Iron-Mediated Lysosomal Membrane Permeabilization in Ethanol-Induced Hepatic Oxidative Damage and Apoptosis: Protective Effects of Quercetin
- PMID: 27057276
- PMCID: PMC4707336
- DOI: 10.1155/2016/4147610
Iron-Mediated Lysosomal Membrane Permeabilization in Ethanol-Induced Hepatic Oxidative Damage and Apoptosis: Protective Effects of Quercetin
Abstract
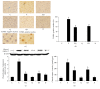
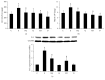
Iron, in its free ferrous states, can catalyze Fenton reaction to produce OH∙, which is recognized as a crucial role in the pathogenesis of alcoholic liver diseases (ALD). As a result of continuous decomposition of iron-containing compounds, lysosomes contain a pool of redox-active iron. To investigate the important role of intralysosomal iron in alcoholic liver injury and the potential protection of quercetin, male C57BL/6J mice fed by Lieber De Carli diets containing ethanol (30% of total calories) were cotreated by quercetin or deferoxamine (DFO) for 15 weeks and ethanol-incubated mice primary hepatocytes were pretreated with FeCl3, DFO, and bafilomycin A1 at their optimal concentrations and exposure times. Chronic ethanol consumption caused an evident increase in lysosomal redox-active iron accompanying sustained oxidative damage. Iron-mediated ROS could trigger lysosomal membrane permeabilization (LMP) and subsequent mitochondria apoptosis. The hepatotoxicity was attenuated by reducing lysosomal iron while being exacerbated by escalating lysosomal iron. Quercetin substantially alleviated the alcoholic liver oxidative damage and apoptosis by decreasing lysosome iron and ameliorating iron-mediated LMP, which provided a new prospective of the use of quercetin against ALD.
Figures




Similar articles
-
Quercetin prevents ethanol-induced iron overload by regulating hepcidin through the BMP6/SMAD4 signaling pathway.J Nutr Biochem. 2014 Jun;25(6):675-82. doi: 10.1016/j.jnutbio.2014.02.009. Epub 2014 Mar 19. J Nutr Biochem. 2014. PMID: 24746831
-
Quercetin protects rat hepatocytes from oxidative damage induced by ethanol and iron by maintaining intercellular liable iron pool.Hum Exp Toxicol. 2014 May;33(5):534-41. doi: 10.1177/0960327113499168. Epub 2013 Aug 8. Hum Exp Toxicol. 2014. PMID: 23928830
-
Refining the Rab7-V1G1 axis to mitigate iron deposition: Protective effects of quercetin in alcoholic liver disease.J Nutr Biochem. 2025 Jan;135:109767. doi: 10.1016/j.jnutbio.2024.109767. Epub 2024 Sep 14. J Nutr Biochem. 2025. PMID: 39284533
-
Lysosomal iron, iron chelation, and cell death.Antioxid Redox Signal. 2013 Mar 10;18(8):888-98. doi: 10.1089/ars.2012.4885. Epub 2012 Oct 9. Antioxid Redox Signal. 2013. PMID: 22909065 Review.
-
Redox activity within the lysosomal compartment: implications for aging and apoptosis.Antioxid Redox Signal. 2010 Aug 15;13(4):511-23. doi: 10.1089/ars.2009.3005. Antioxid Redox Signal. 2010. PMID: 20039839 Review.
Cited by
-
Reactive Oxygen Species as Key Molecules in the Pathogenesis of Alcoholic Fatty Liver Disease and Nonalcoholic Fatty Liver Disease: Future Perspectives.Curr Issues Mol Biol. 2025 Jun 17;47(6):464. doi: 10.3390/cimb47060464. Curr Issues Mol Biol. 2025. PMID: 40699863 Free PMC article. Review.
-
ER-to-lysosome Ca2+ refilling followed by K+ efflux-coupled store-operated Ca2+ entry in inflammasome activation and metabolic inflammation.Elife. 2024 Jul 2;12:RP87561. doi: 10.7554/eLife.87561. Elife. 2024. PMID: 38953285 Free PMC article.
-
Alcohol-Induced Lysosomal Damage and Suppression of Lysosome Biogenesis Contribute to Hepatotoxicity in HIV-Exposed Liver Cells.Biomolecules. 2021 Oct 11;11(10):1497. doi: 10.3390/biom11101497. Biomolecules. 2021. PMID: 34680130 Free PMC article.
-
Metabolic Derangement of Essential Transition Metals and Potential Antioxidant Therapies.Int J Mol Sci. 2024 Jul 18;25(14):7880. doi: 10.3390/ijms25147880. Int J Mol Sci. 2024. PMID: 39063122 Free PMC article. Review.
-
Iron Promotes Dihydroartemisinin Cytotoxicity via ROS Production and Blockade of Autophagic Flux via Lysosomal Damage in Osteosarcoma.Front Pharmacol. 2020 May 5;11:444. doi: 10.3389/fphar.2020.00444. eCollection 2020. Front Pharmacol. 2020. PMID: 32431605 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

