A Case-Control Study of Involvement of Oxidative DNA Damage and Alteration of Antioxidant Defense System in Patients with Basal Cell Carcinoma: Modulation by Tumor Removal
- PMID: 27057281
- PMCID: PMC4738719
- DOI: 10.1155/2016/5934024
A Case-Control Study of Involvement of Oxidative DNA Damage and Alteration of Antioxidant Defense System in Patients with Basal Cell Carcinoma: Modulation by Tumor Removal
Abstract
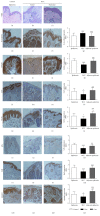
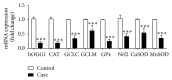
Oxidative damage has been suggested to play a role in the pathogenesis of basal cell carcinoma (BCC). This study illustrated an involvement of oxidative DNA damage and changes in antioxidant defenses in BCC by conducting a case-control study (24 controls and 24 BCC patients) and assessing urinary 7,8-dihydro-8-oxo-2'-deoxyguanosine (8-oxo-dGuo), plasma antioxidant defenses including catalase (CAT), glutathione peroxidase (GPx), NQO1, and total superoxide dismutase (SOD) activities, and glutathione (GSH) levels before surgery and 1 month after surgery. 8-oxo-dGuo expressions as well as protein and mRNA expressions of DNA repair enzyme hOGG1 and antioxidant defenses (CAT, GCLC, GPx, Nrf2, and MnSOD) in nonneoplastic epidermis of control and BCC tissues were also determined. This study observed induction in urinary 8-oxo-dGuo, increased 8-oxo-dGuo expression, and reduced hOGG1 protein and mRNA in BCC tissues, decreased activities of CAT, GPx, and NQO1, but elevated SOD activities and GSH levels in BCC patients and reduction of all antioxidant proteins and genes studied in BCC tissues. Furthermore, decreased plasma antioxidant activities in BCC patients were restored at 1 month after operation compared with preoperative levels. Herein, we concluded that BCC patients were associated with oxidative DNA damage and depletion of antioxidant defenses and surgical removal of BCC correlated with improved redox status.
Figures

Similar articles
-
Human 8-oxoguanine-DNA glycosylase-1 is downregulated in human basal cell carcinoma.Mol Genet Metab. 2012 May;106(1):127-30. doi: 10.1016/j.ymgme.2012.02.017. Epub 2012 Mar 3. Mol Genet Metab. 2012. PMID: 22436579
-
Oxidative damage and antioxidant defense in thymus of malnourished lactating rats.Nutrition. 2015 Nov-Dec;31(11-12):1408-15. doi: 10.1016/j.nut.2015.05.014. Epub 2015 Jun 6. Nutrition. 2015. PMID: 26429663
-
Oxidative stress and 8-oxoguanine repair are enhanced in colon adenoma and carcinoma patients.Mutagenesis. 2010 Sep;25(5):463-71. doi: 10.1093/mutage/geq028. Epub 2010 Jun 9. Mutagenesis. 2010. PMID: 20534734
-
Exogenous 8-Oxo-7,8-dihydro-2'-deoxyguanosine: Biomedical Properties, Mechanisms of Action, and Therapeutic Potential.Biochemistry (Mosc). 2017 Dec;82(13):1686-1701. doi: 10.1134/S0006297917130089. Biochemistry (Mosc). 2017. PMID: 29523066 Review.
-
Redox-regulation of DNA repair.Biofactors. 2003;17(1-4):315-24. doi: 10.1002/biof.5520170131. Biofactors. 2003. PMID: 12897453 Review. No abstract available.
Cited by
-
Clinicopathologic and Molecular Characterization of Basal Cell Carcinoma Arising at Sun-protected Sites.Am J Surg Pathol. 2025 Apr 1;49(4):328-335. doi: 10.1097/PAS.0000000000002366. Epub 2025 Jan 14. Am J Surg Pathol. 2025. PMID: 39807820 Free PMC article.
-
Emerging Perspective: Role of Increased ROS and Redox Imbalance in Skin Carcinogenesis.Oxid Med Cell Longev. 2019 Sep 16;2019:8127362. doi: 10.1155/2019/8127362. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31636809 Free PMC article. Review.
-
Approaches and Methods to Measure Oxidative Stress in Clinical Samples: Research Applications in the Cancer Field.Oxid Med Cell Longev. 2019 Mar 12;2019:1279250. doi: 10.1155/2019/1279250. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 30992736 Free PMC article. Review.
-
Hydrochlorothiazide Use and Risk of Nonmelanoma Skin Cancers: A Biological Plausibility Study.Oxid Med Cell Longev. 2021 Aug 14;2021:6655542. doi: 10.1155/2021/6655542. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34434485 Free PMC article.
-
Ultraviolet Radiation-Induced Skin Aging: The Role of DNA Damage and Oxidative Stress in Epidermal Stem Cell Damage Mediated Skin Aging.Stem Cells Int. 2016;2016:7370642. doi: 10.1155/2016/7370642. Epub 2016 Apr 11. Stem Cells Int. 2016. PMID: 27148370 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

