Dose and time response of ruminally infused algae on rumen fermentation characteristics, biohydrogenation and Butyrivibrio group bacteria in goats
- PMID: 27057310
- PMCID: PMC4823909
- DOI: 10.1186/s40104-016-0080-1
Dose and time response of ruminally infused algae on rumen fermentation characteristics, biohydrogenation and Butyrivibrio group bacteria in goats
Abstract
Background: Micro-algae could inhibit the complete rumen BH of dietary 18-carbon unsaturated fatty acid (UFAs). This study aimed to examine dose and time responses of algae supplementation on rumen fermentation, biohydrogenation and Butyrivibrio group bacteria in goats.
Methods: Six goats were used in a repeated 3 × 3 Latin square design, and offered a fixed diet. Algae were infused through rumen cannule with 0 (Control), 6.1 (L-Alg), or 18.3 g (H-Alg) per day. Rumen contents were sampled on d 0, 3, 7, 14 and 20.
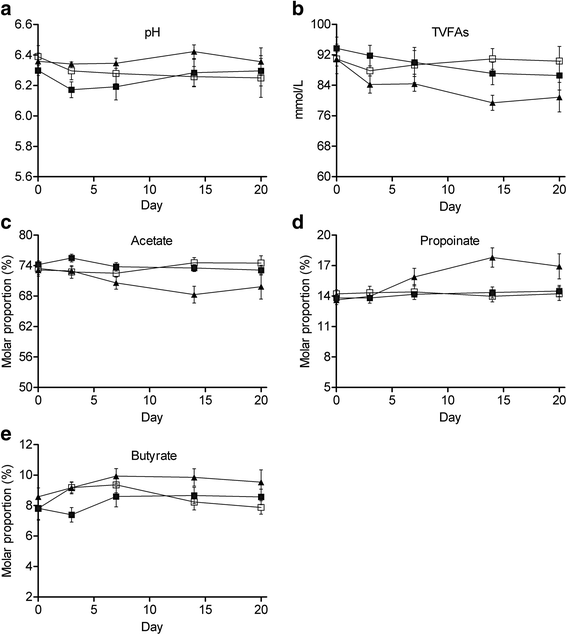
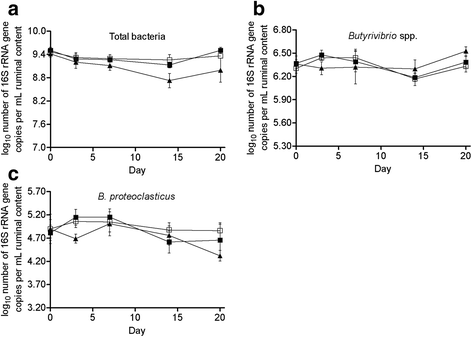
Results: H-Alg reduced total volatile fatty acid concentration and acetate molar proportion (P < 0.05), and increased propionate molar proportion (P < 0.05), whereas L-Alg had no effect on rumen fermentation. Changes in proportions of acetate and propionate in H-Alg were obvious from d 7 onwards and reached the largest differences with the control on d 14. Algae induced a dose-dependent decrease in 18:0 and increased trans-18:1 in the ruminal content (P < 0.05). H-Alg increased the concentrations of t9, t11-18:2 and t11, c15-18:2 (P < 0.05). L-Alg only seemed to induce a transient change in 18-carbon isomers, while H-Alg induced a rapid elevation, already obvious on d 3, concentrations of these fatty acid rose in some cases again on d 20. Algae had no effect on the abundances of Butyrivibrio spp. and Butyrivibrio proteoclasticus (P > 0.10), while H-Alg reduced the total bacteria abundance (P < 0.05). However, this was induced by a significant difference between control and H-Alg on d 14 (-4.43 %). Afterwards, both treatments did not differ as increased variation in the H-Alg repetitions, with in some cases a return of the bacterial abundance to the basal level (d 0).
Conclusions: Changes in rumen fermentation and 18-carbon UFAs metabolism in response to algae were related to the supplementation level, but there was no evidence of shift in ruminal biohydrogenation pathways towards t10-18:1. L-Alg mainly induced a transient effect on rumen biohydrogenation of 18-carbon UFAs, while H-Alg showed an acute inhibition and these effects were not associated with the known hydrogenating bacteria.
Keywords: Algae; Biohydrogenation; Goat; Hydrogenating bacteria.
Figures

Similar articles
-
Impairment of rumen biohydrogenation and bacteria of the Butyrivibrio group in the rumen of goats through a 20:5 n-3 (EPA) rich supplement.J Sci Food Agric. 2016 Jan 30;96(2):474-83. doi: 10.1002/jsfa.7113. Epub 2015 Mar 3. J Sci Food Agric. 2016. PMID: 25639507 Clinical Trial.
-
Effects of ruminal infusion of garlic oil on fermentation dynamics, Fatty Acid profile and abundance of bacteria involved in biohydrogenation in rumen of goats.Asian-Australas J Anim Sci. 2012 Jul;25(7):962-70. doi: 10.5713/ajas.2011.11442. Asian-Australas J Anim Sci. 2012. PMID: 25049651 Free PMC article.
-
Accumulation of trans C18:1 fatty acids in the rumen after dietary algal supplementation is associated with changes in the Butyrivibrio community.Appl Environ Microbiol. 2008 Nov;74(22):6923-30. doi: 10.1128/AEM.01473-08. Epub 2008 Sep 26. Appl Environ Microbiol. 2008. PMID: 18820074 Free PMC article.
-
Fatty acid composition and bacterial community changes in the rumen fluid of lactating sheep fed sunflower oil plus incremental levels of marine algae.J Dairy Sci. 2012 Feb;95(2):794-806. doi: 10.3168/jds.2011-4561. J Dairy Sci. 2012. PMID: 22281344
-
Invited review: Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: Experimental evidence and methodological approaches.J Dairy Sci. 2019 May;102(5):3781-3804. doi: 10.3168/jds.2018-14985. Epub 2019 Mar 21. J Dairy Sci. 2019. PMID: 30904293 Review.
Cited by
-
Freeze-dried Nannochloropsis oceanica biomass protects eicosapentaenoic acid (EPA) from metabolization in the rumen of lambs.Sci Rep. 2021 Nov 8;11(1):21878. doi: 10.1038/s41598-021-01255-w. Sci Rep. 2021. PMID: 34750444 Free PMC article.
-
Rumen Biohydrogenation and Microbial Community Changes Upon Early Life Supplementation of 22:6n-3 Enriched Microalgae to Goats.Front Microbiol. 2018 Mar 27;9:573. doi: 10.3389/fmicb.2018.00573. eCollection 2018. Front Microbiol. 2018. PMID: 29636742 Free PMC article.
-
Identifying and exploring biohydrogenating rumen bacteria with emphasis on pathways including trans-10 intermediates.BMC Microbiol. 2020 Jul 7;20(1):198. doi: 10.1186/s12866-020-01876-7. BMC Microbiol. 2020. PMID: 32635901 Free PMC article.
-
Role of Dietary Inclusion of Phytobiotics and Mineral Adsorbent Combination on Dairy Cows' Milk Production, Nutrient Digestibility, Nitrogen Utilization, and Biochemical Parameters.Vet Sci. 2023 Mar 22;10(3):238. doi: 10.3390/vetsci10030238. Vet Sci. 2023. PMID: 36977277 Free PMC article.
-
Rumen bacterial community responses to DPA, EPA and DHA in cattle and sheep: A comparative in vitro study.Sci Rep. 2019 Aug 14;9(1):11857. doi: 10.1038/s41598-019-48294-y. Sci Rep. 2019. PMID: 31413283 Free PMC article.
References
-
- Harfoot CG, Hazlewood GP. Lipid metabolism in the rumen. In: Hobson PN, Stewart CS, editors. The Rumen Microbial Ecosystem. London: Chapman & Hall; 1997. pp. 382–426.
-
- Chilliard Y, Glasser F, Ferlay A, Bernard L, Rouel J, Doreau M. Diet, rumen biohydrogenation and nutritional quality of cow and goat milk fat. Eur J Lipid Sci Technol. 2007;109:828–55. doi: 10.1002/ejlt.200700080. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

