Phenotype, development, and biological function of myeloid-derived suppressor cells
- PMID: 27057424
- PMCID: PMC4801459
- DOI: 10.1080/2162402X.2015.1004983
Phenotype, development, and biological function of myeloid-derived suppressor cells
Abstract
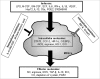
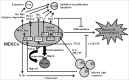
CD11b+Gr-1+ myeloid-derived suppressor cells (MDSCs) are an important population of innate regulatory cells mainly comprising monocytic MDSCs (M-MDSCs) with a phenotype of CD11b+Ly6G-Ly6Chigh and granulocytic MDSCs (G-MDSCs) with a phenotype of CD11b+Ly6G+Ly6Clow in mice. They play crucial roles in the pathogenesis of cancers, chronic infections, autoimmune diseases, and transplantation. Various extracellular factors such as lipopolysaccharide (LPS), macrophage colony-stimulating factor (M-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), stem cell factor (SCF), interleukin (IL)-6, interferon gamma (IFNγ), IL-1β, vascular endothelial growth factor (VEGF), Hsp72, IL-13, C5a, and prostaglandin E2 (PGE2) can induce MDSC differentiation, whereas IL-4 and all-trans-retinoic acid can inhibit this process. For the intracellular signals, signal transducer and activator of transcription (STAT) family members, C/EBPβ and cyclooxigenase-2 (COX-2) promote MDSC function, whereas interferon regulatory factor-8 (IRF-8) and Smad3 downregulate MDSC activity. The immunosuppressive function of MDSCs is mediated through various effector molecules, primarily cellular metabolism-related molecules such as nitric oxide (NO), arginase, reactive oxygen species (ROS), transforming growth factor β (TGFβ), IL-10, indoleamine 2,3-dioxygenase (IDO), heme oxygenase-1 (HO-1), carbon monoxide (CO), and PGE2. In this article, we will summarize the molecules involved in the induction and function of MDSCs as well as the regulatory pathways of MDSCs.
Keywords: immune modulation; immune tolerance; immunosuppression; innate cells; myeloid-derived suppressor cells.
Figures
References
-
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162-74; PMID:19197294; http://dx.doi.org/10.1038/nri2506 - DOI - PMC - PubMed
-
- Schleifer KW, Mansfield JM. Suppressor macrophages in African trypanosomiasis inhibit T cell proliferative responses by nitric oxide and prostaglandins. J Immunol 1993; 151:5492-503; PMID:8228241 - PubMed
-
- Haskill S, Koren H, Becker S, Fowler W, Walton L. Mononuclear-cell infiltration in ovarian cancer. III. Suppressor-cell and ADCC activity of macrophages from ascitic and solid ovarian tumours. Br J Cancer 1982; 45:747-53; PMID:6211187; http://dx.doi.org/10.1038/bjc.1982.116 - DOI - PMC - PubMed
-
- Pak AS, Wright MA, Matthews JP, Collins SL, Petruzzelli GK, Young MR. Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin Cancer Res 1995; 1:95-103; PMID:9815891 - PubMed
-
- Mishra PK, Morris EG, Garcia JA, Cardona AE, Teale JM. Increased accumulation of regulatory granulocytic myeloid cells in mannose receptor C type 1-deficient mice correlates with protection in a mouse model of neurocysticercosis. Infect Immun 2013; 81:1052-63; PMID:23319563; http://dx.doi.org/10.1128/IAI.01176-12 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
