Differential effects of lenalidomide during plasma cell differentiation
- PMID: 27057635
- PMCID: PMC5053712
- DOI: 10.18632/oncotarget.8581
Differential effects of lenalidomide during plasma cell differentiation
Abstract
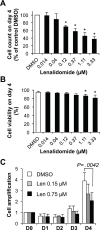
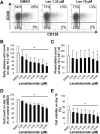
Thalidomide, lenalidomide and pomalidomide have greatly improved the outcome of patients with multiple myeloma. However, their effects on plasma cells, the healthy counterpart of myeloma cells, are unknown. Here, we investigated lenalidomide effects on normal human plasma cell generation using an in vitro model. Lenalidomide inhibited the generation of pre-plasmablasts and early plasma cells, while it moderately affected plasmablast production. It also reduced the expression level of Ikaros, Aiolos, and IRF4 transcription factors, in plasmablasts and early plasma cells. This suggests that their differential sensitivity to lenalidomide is not due to a difference in Ikaros or Aiolos degradation. Lenalidomide also inhibited long-lived plasma cell generation, but did not impair their long-term survival once generated. This last observation is in agreement with the finding that lenalidomide treatment for 3-18 months did not affect the bone marrow healthy plasma cell count in allografted patients with multiple myeloma. Our findings should prompt to investigate whether lenalidomide resistance in patients with multiple myeloma could be associated with the emergence of malignant plasmablasts or long-lived plasma cells that are less sensitive to lenalidomide.
Keywords: IKZF1; IKZF3; differentiation; lenalidomide; plasma cell.
Conflict of interest statement
This study was partly supported by research funding from Celgene Corporation. RC and PS are employees of Celgene and hold stock options in Celgene. GC has received honoraria from Celgene. The remaining authors declare no conflict of interest.
Figures







References
-
- Barlogie B, Desikan R, Eddlemon P, Spencer T, Zeldis J, Munshi N, Badros A, Zangari M, Anaissie E, Epstein J, Shaughnessy J, Ayers D, Spoon D, Tricot G. Extended survival in advanced and refractory multiple myeloma after single-agent thalidomide: identification of prognostic factors in a phase 2 study of 169 patients. Blood. 2001;98:492–4. - PubMed
-
- Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, Stoppa AM, Hulin C, Benboubker L, Garderet L, Decaux O, Leyvraz S, Vekemans MC, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1782–91. - PubMed
-
- Lacy MQ, McCurdy AR. Pomalidomide. Blood. 2013;122:2305–9. - PubMed
-
- Shortt J, Hsu AK, Johnstone RW. Thalidomide-analogue biology: immunological, molecular and epigenetic targets in cancer therapy. Oncogene. 2013;32:4191–202. - PubMed
-
- Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, Yamaguchi Y, Handa H. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–50. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

