Are Everolimus-Eluting Stents Associated With Better Clinical Outcomes Compared to Other Drug-Eluting Stents in Patients With Type 2 Diabetes Mellitus?: A Systematic Review and Meta-Analysis
- PMID: 27057888
- PMCID: PMC4998804
- DOI: 10.1097/MD.0000000000003276
Are Everolimus-Eluting Stents Associated With Better Clinical Outcomes Compared to Other Drug-Eluting Stents in Patients With Type 2 Diabetes Mellitus?: A Systematic Review and Meta-Analysis
Abstract
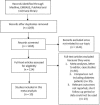
Controversies still exist with the use of Everolimus-Eluting Stents (EES) compared to other Drug-Eluting Stents (DES) in patients with Type 2 Diabetes Mellitus (T2DM). Therefore, in order to solve this issue, we aim to compare the 1-year adverse clinical outcomes between EES and non-EE DES with a larger number of patients with T2DM.Medline, EMBASE, PubMed databases, as well as the Cochrane library were searched for randomized controlled trials (RCTs) and observational studies (OS) comparing EES and non-EE DES in patients with T2DM. One-year adverse outcomes were considered as the clinical endpoints in this study. Odd ratios (OR) with 95% confidence interval (CI) were used to express the pooled effect on discontinuous variables and the pooled analyses were performed with RevMan 5.3.Ten studies consisting of a total of 11,981 patients with T2DM (6800 patients in the EES group and 5181 in the non-EE DES group) were included in this meta-analysis. EES were associated with a significantly lower major adverse cardiac events (MACEs) with OR: 0.83, 95% CI: 0.70-0.98, P = 0.03. Revascularization including target vessel revascularization (TVR) and target lesion revascularization (TLR) were also significantly lower in the EES group with OR: 0.62, 95% CI: 0.40-0.94, P = 0.03 and OR: 0.74, 95% CI: 0.57-0.95, P = 0.02, respectively. Also, a significantly lower rate of stent thrombosis with OR: 0.63, 95% CI: 0.46-0.86, P = 0.003 was observed in the EES group. However, a similar mortality rate was reported between the EES and non-EE DES groups.During this 1-year follow-up period, EES were associated with significantly better clinical outcomes compared to non-EE DES in patients suffering from T2DM. However, further research comparing EES with non-EE DES in insulin-treated and noninsulin-treated patients with T2DM are recommended.
Conflict of interest statement
No writing assistance was required and the authors declare no competing interests. The authors have no conflicts of interest to disclose.
Figures



Similar articles
-
Stent thrombosis and adverse cardiovascular outcomes observed between six months and five years with sirolimus-eluting stents and other drug-eluting stents in patients with Type 2 diabetes mellitus complicated by coronary artery disease: A systematic review and meta-analysis.Medicine (Baltimore). 2016 Jul;95(27):e4130. doi: 10.1097/MD.0000000000004130. Medicine (Baltimore). 2016. PMID: 27399125 Free PMC article.
-
Comparing Stent Thrombosis associated with Zotarolimus Eluting Stents versus Everolimus Eluting Stents at 1 year follow up: a systematic review and meta-analysis of 6 randomized controlled trials.BMC Cardiovasc Disord. 2017 Mar 16;17(1):84. doi: 10.1186/s12872-017-0515-4. BMC Cardiovasc Disord. 2017. PMID: 28302055 Free PMC article.
-
Comparing the Clinical Outcomes between Drug Eluting Stents and Bare Metal Stents in Patients with Insulin-Treated Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of 10 Randomized Controlled Trials.PLoS One. 2016 Apr 25;11(4):e0154064. doi: 10.1371/journal.pone.0154064. eCollection 2016. PLoS One. 2016. PMID: 27111304 Free PMC article.
-
Adverse cardiovascular events associated with biodegradable polymer drug-eluting stents and durable polymer everolimus-eluting stents: A systematic review and meta-analysis of 10 randomized controlled trials.Medicine (Baltimore). 2017 Jul;96(28):e7510. doi: 10.1097/MD.0000000000007510. Medicine (Baltimore). 2017. PMID: 28700502 Free PMC article.
-
Clinical outcomes with drug-eluting and bare-metal stents in patients with ST-segment elevation myocardial infarction: evidence from a comprehensive network meta-analysis.J Am Coll Cardiol. 2013 Aug 6;62(6):496-504. doi: 10.1016/j.jacc.2013.05.022. Epub 2013 Jun 7. J Am Coll Cardiol. 2013. PMID: 23747778
Cited by
-
Early (≤ 30 Days), Late (31-360 Days) and Very Late (> 360 Days) Stent Thrombosis in Patients with Insulin-Treated versus Non-Insulin-Treated Type 2 Diabetes Mellitus: A Meta-Analysis.Diabetes Ther. 2018 Jun;9(3):1113-1124. doi: 10.1007/s13300-018-0425-1. Epub 2018 Apr 11. Diabetes Ther. 2018. PMID: 29644619 Free PMC article.
-
Stent thrombosis and adverse cardiovascular outcomes observed between six months and five years with sirolimus-eluting stents and other drug-eluting stents in patients with Type 2 diabetes mellitus complicated by coronary artery disease: A systematic review and meta-analysis.Medicine (Baltimore). 2016 Jul;95(27):e4130. doi: 10.1097/MD.0000000000004130. Medicine (Baltimore). 2016. PMID: 27399125 Free PMC article.
-
Adverse Cardiovascular Outcomes associated with Coronary Artery Bypass Surgery and Percutaneous Coronary Intervention with Everolimus Eluting Stents: A Meta-Analysis.Sci Rep. 2016 Oct 24;6:35869. doi: 10.1038/srep35869. Sci Rep. 2016. PMID: 27775055 Free PMC article.
-
Duration of Dual Antiplatelet Therapy and Late Stent Thrombosis Following Percutaneous Coronary Intervention with Second-Generation Drug-Eluting Stents: A Simple Meta-Analysis of Randomized Controlled Trials.Adv Ther. 2019 Nov;36(11):3166-3173. doi: 10.1007/s12325-019-01091-5. Epub 2019 Sep 18. Adv Ther. 2019. PMID: 31535329 Free PMC article.
-
Comparing Stent Thrombosis associated with Zotarolimus Eluting Stents versus Everolimus Eluting Stents at 1 year follow up: a systematic review and meta-analysis of 6 randomized controlled trials.BMC Cardiovasc Disord. 2017 Mar 16;17(1):84. doi: 10.1186/s12872-017-0515-4. BMC Cardiovasc Disord. 2017. PMID: 28302055 Free PMC article.
References
-
- Sabaté M, Brugaletta S, Cequier A, et al. The EXAMINATION trial (Everolimus-Eluting Stents Versus Bare-Metal Stents in ST-Segment ElevationMyocardial Infarction): 2-year results from a multicenter randomized controlled trial. JACC Cardiovasc Interv 2014; 7:64–71. - PubMed
-
- Stone GW, Lansky AJ, Pocock SJ, et al. HORIZONS-AMI Trial Investigators. Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N Engl J Med 2009; 360:1946–1959. - PubMed
-
- Di Lorenzo E, De Luca G, Sauro R, et al. The PASEO (PaclitAxel or Sirolimus-Eluting Stent Versus Bare Metal Stent in Primary Angioplasty) randomized trial. JACC Cardiovasc Interv 2009; 2:515–523. - PubMed
-
- Baber U, Mehran R, Sharma SK, et al. Impact of the everolimus-eluting stent on stent thrombosis: a meta-analysis of 13 randomized trials. J Am Coll Cardiol 2011; 58:1569–1577. - PubMed
-
- Mathew V, Gersh BJ, Williams BA, et al. Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: a report from the Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation 2004; 109:476–480. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

