Histone Deacetylase Inhibitors Trichostatin A and MCP30 Relieve Benzene-Induced Hematotoxicity via Restoring Topoisomerase IIα
- PMID: 27058040
- PMCID: PMC4826000
- DOI: 10.1371/journal.pone.0153330
Histone Deacetylase Inhibitors Trichostatin A and MCP30 Relieve Benzene-Induced Hematotoxicity via Restoring Topoisomerase IIα
Abstract
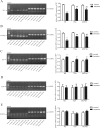
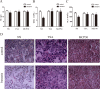
Dysfunction of histone acetylation inhibits topoisomerase IIα (Topo IIα), which is implicated in benzene-induced hematotoxicity in patients with chronic benzene exposure. Whether histone deacetylase (HDAC) inhibitors can relieve benzene-induced hematotoxicity remains unclear. Here we showed that hydroquinone, a main metabolite of benzene, increased the HDAC activity, decreased the Topo IIα expression and induced apoptosis in human bone marrow mononuclear cells in vitro, and treatment with two HDAC inhibitors, namely trichostatin A (TSA) or a mixture of ribosome-inactivating proteins MCP30, almost completely reversed these effects. We further established a benzene poisoning murine model by inhaling benzene vapor in a container and found that benzene poisoning decreased the expression and activity of Topo IIα, and impaired acetylation of histone H4 and H3. The analysis of regulatory factors of Topo IIα promoter found that benzene poisoning decreased the mRNA levels of SP1 and C-MYB, and increased the mRNA level of SP3. Both TSA and MCP30 significantly enhanced the acetylation of histone H3 and H4 in Topo IIα promoter and increased the expression and activity of Topo IIα in benzene poisoning mice, which contributed to relieve the symptoms of hematotoxicity. Thus, treatment with HDAC inhibitors represents an attractive approach to reduce benzene-induced hematotoxicity.
Conflict of interest statement
Figures



References
-
- Faiola B, Fuller ES, Wong VA, Pluta L, Abernethy DJ, Rose J, et al. Exposure of hematopoietic stem cells to benzene or 1,4-benzoquinone induces gender-specific gene expression. Stem Cells. 2004; 22: 750–758. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

