Unilateral microinjection of acrolein into thoracic spinal cord produces acute and chronic injury and functional deficits
- PMID: 27058147
- PMCID: PMC4854649
- DOI: 10.1016/j.neuroscience.2016.03.054
Unilateral microinjection of acrolein into thoracic spinal cord produces acute and chronic injury and functional deficits
Abstract
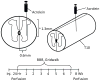
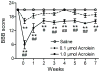
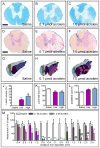
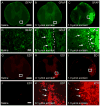
Although lipid peroxidation has long been associated with spinal cord injury (SCI), the specific role of lipid peroxidation-derived byproducts such as acrolein in mediating damage remains to be fully understood. Acrolein, an α-β unsaturated aldehyde, is highly reactive with proteins, DNA, and phospholipids and is considered as a second toxic messenger that disseminates and augments initial free radical events. Previously, we showed that acrolein increased following traumatic SCI and injection of acrolein induced tissue damage. Here, we demonstrate that microinjection of acrolein into the thoracic spinal cord of adult rats resulted in dose-dependent tissue damage and functional deficits. At 24h (acute) after the microinjection, tissue damage, motoneuron loss, and spinal cord swelling were observed on sections stained with Cresyl Violet. Luxol fast blue staining further showed that acrolein injection resulted in dose-dependent demyelination. At 8weeks (chronic) after the microinjection, cord shrinkage, astrocyte activation, and macrophage infiltration were observed along with tissue damage, neuron loss, and demyelination. These pathological changes resulted in behavioral impairments as measured by both the Basso, Beattie, and Bresnahan (BBB) locomotor rating scale and grid walking analysis. Electron microscopy further demonstrated that acrolein induced axonal degeneration, demyelination, and macrophage infiltration. These results, combined with our previous reports, strongly suggest that acrolein may play a critical causal role in the pathogenesis of SCI and that targeting acrolein could be an attractive strategy for repair after SCI.
Keywords: acrolein; aldehyde; lipid peroxidation; oxidative stress; spinal cord injury.
Copyright © 2016 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures



References
-
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. - PubMed
-
- Behrmann DL, Bresnahan JC, Beattie MS, Shah BR. Spinal cord injury produced by consistent mechanical displacement of the cord in rats: behavioral and histologic analysis. J Neurotrauma. 1992;9:197–217. - PubMed
-
- Blight AR. Delayed demyelination and macrophage invasion: a candidate for secondary cell damage in spinal cord injury. Cent Nerv Syst Trauma. 1985;2:299–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

