Metabolic reprogramming during neuronal differentiation
- PMID: 27058317
- PMCID: PMC5072427
- DOI: 10.1038/cdd.2016.36
Metabolic reprogramming during neuronal differentiation
Abstract
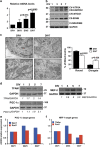
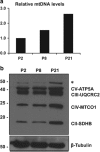
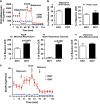
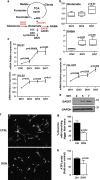
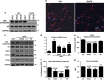
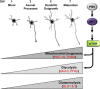
Newly generated neurons pass through a series of well-defined developmental stages, which allow them to integrate into existing neuronal circuits. After exit from the cell cycle, postmitotic neurons undergo neuronal migration, axonal elongation, axon pruning, dendrite morphogenesis and synaptic maturation and plasticity. Lack of a global metabolic analysis during early cortical neuronal development led us to explore the role of cellular metabolism and mitochondrial biology during ex vivo differentiation of primary cortical neurons. Unexpectedly, we observed a huge increase in mitochondrial biogenesis. Changes in mitochondrial mass, morphology and function were correlated with the upregulation of the master regulators of mitochondrial biogenesis, TFAM and PGC-1α. Concomitant with mitochondrial biogenesis, we observed an increase in glucose metabolism during neuronal differentiation, which was linked to an increase in glucose uptake and enhanced GLUT3 mRNA expression and platelet isoform of phosphofructokinase 1 (PFKp) protein expression. In addition, glutamate-glutamine metabolism was also increased during the differentiation of cortical neurons. We identified PI3K-Akt-mTOR signalling as a critical regulator role of energy metabolism in neurons. Selective pharmacological inhibition of these metabolic pathways indicate existence of metabolic checkpoint that need to be satisfied in order to allow neuronal differentiation.
Figures


References
-
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 2001; 21: 1133–1145. - PubMed
-
- McKenna MC. The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in brain. J Neurosci Res 2007; 85: 3347–3358. - PubMed
-
- Erecinska M, Zaleska MM, Nissim I, Nelson D, Dagani F, Yudkoff M. Glucose and synaptosomal glutamate metabolism: studies with [15 N]glutamate. J Neurochem 1988; 51: 892–902. - PubMed
-
- Sonnewald U, McKenna M. Metabolic compartmentation in cortical synaptosomes: influence of glucose and preferential incorporation of endogenous glutamate into GABA. Neurochem Res 2002; 27: 43–50. - PubMed
-
- Chang DT, Reynolds IJ. Differences in mitochondrial movement and morphology in young and mature primary cortical neurons in culture. Neuroscience 2006; 141: 727–736. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

