Assessment of physical function and participation in chronic pain clinical trials: IMMPACT/OMERACT recommendations
- PMID: 27058676
- PMCID: PMC7453823
- DOI: 10.1097/j.pain.0000000000000577
Assessment of physical function and participation in chronic pain clinical trials: IMMPACT/OMERACT recommendations
Abstract
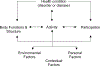
Although pain reduction is commonly the primary outcome in chronic pain clinical trials, physical functioning is also important. A challenge in designing chronic pain trials to determine efficacy and effectiveness of therapies is obtaining appropriate information about the impact of an intervention on physical function. The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) and Outcome Measures in Rheumatology (OMERACT) convened a meeting to consider assessment of physical functioning and participation in research on chronic pain. The primary purpose of this article is to synthesize evidence on the scope of physical functioning to inform work on refining physical function outcome measurement. We address issues in assessing this broad construct and provide examples of frequently used measures of relevant concepts. Investigators can assess physical functioning using patient-reported outcome (PRO), performance-based, and objective measures of activity. This article aims to provide support for the use of these measures, covering broad aspects of functioning, including work participation, social participation, and caregiver burden, which researchers should consider when designing chronic pain clinical trials. Investigators should consider the inclusion of both PROs and performance-based measures as they provide different but also important complementary information. The development and use of reliable and valid PROs and performance-based measures of physical functioning may expedite development of treatments, and standardization of these measures has the potential to facilitate comparison across studies. We provide recommendations regarding important domains to stimulate research to develop tools that are more robust, address consistency and standardization, and engage patients early in tool development.
Conflict of interest statement
The views expressed in this article are those of the authors, none of whom have financial conflicts of interest relevant to the issues discussed in this manuscript.
Figures

References
-
- Agarwal S, Polydefkis M, Block B, Haythornthwaite J, Raja SN. Transdermal fentanyl reduces pain and improves functional activity in neuropathic pain states. Pain Med 2007;8:554–62. - PubMed
-
- Alghwiri AA, Whitney SL, Baker CE, Sparto PJ, Marchetti GF, Rogers JC, Furman JM. The development and validation of the vestibular activities and participation measure. Arch Phys Med Rehabil 2012;93:1822–31. - PubMed
-
- Alonso J, Bartlett SJ, Rose M, Aaronson NK, Chaplin JE, Efficace F, Leplege A, Lu A, Tulsky DS, Raat H, Ravens-Sieberer U, Revicki D, Terwee CB, Valderas JM, Cella D, Forrest CB, Group PI. The case for an international patient-reported outcomes measurement information system (PROMIS(R)) initiative. Health and quality of life outcomes 2013;11:210. - PMC - PubMed
-
- Anagnostis C, Gatchel RJ, Mayer TG. The pain disability questionnaire: a new psychometrically sound measure for chronic musculoskeletal disorders. Spine (Phila Pa 1976) 2004;29:2290–302; discussion 2303. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

