The role of metformin and resveratrol in the prevention of hypoxia-inducible factor 1α accumulation and fibrosis in hypoxic adipose tissue
- PMID: 27059094
- PMCID: PMC4882491
- DOI: 10.1111/bph.13493
The role of metformin and resveratrol in the prevention of hypoxia-inducible factor 1α accumulation and fibrosis in hypoxic adipose tissue
Abstract
Background and purpose: Hypoxic activation of hypoxia-inducible factor 1α (HIF-1α) and fibrosis in adipose tissue contribute to adipose dysfunction. This study was designed to investigate the effects of metformin and resveratrol on the regulation of HIF-1α and fibrosis in hypoxic adipose tissue.
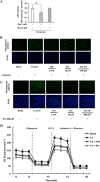
Experimental approach: Mice were fed a high-fat diet to induce hypoxia and fibrosis in adipose tissue; adipose tissue incubated in vitro in 1% O2 showed a similar change. The effects of metformin and resveratrol on hypoxia, HIF-1α accumulation, endoplasmic reticulum stress and gene expressions of extracellular matrix components and pro-inflammatory cytokines were examined.
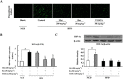
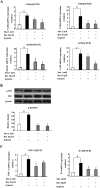
Key results: Oral administration of metformin or resveratrol prevented hypoxia and reduced HIF-1α accumulation with dephosphorylation of inositol-requiring enzyme 1α and eukaryotic initiation factor 2α, indicative of suppression of hypoxic HIF-1α activation and endoplasmic reticulum stress. Metformin and resveratrol down-regulated gene expressions of Col3α, Col6α, elastin and lysyl oxidase and thereby reduced collagen deposition in adipose tissue. The increased gene expressions of TNF-α, IL-6, monocyte chemoattractant protein 1 and F4/80 were also down-regulated by metformin and resveratrol. Metformin and resveratrol had similar effects in adipose tissue exposed to 1% O2 . Metformin reduced ATP production and prevented the reduction in oxygen tension in 3T3-L1 cells, suggesting that it prevented hypoxia by limiting oxygen consumption, whereas resveratrol reduced HIF-1α accumulation by promoting its proteasomal degradation via the regulation of AMPK/SIRT1.
Conclusion and implications: Hypoxia and fibrosis are early causes of adipose dysfunction in obesity. Both metformin and resveratrol effectively inhibited HIF-1α activation-induced fibrosis and inflammation in adipose tissue, although by different mechanisms.
© 2016 The British Pharmacological Society.
Figures






References
-
- Bai Y, Lu H, Wu C, Liang Y, Wang S, Lin C et al. (2014). Resveratrol inhibits epithelial–mesenchymal transition and renal fibrosis by antagonizing the hedgehog signaling pathway. Biochem Pharmacol 92: 484–493. - PubMed
-
- Brasnyó P, Molnár GA, Mohás M, Markóal L, Laczyal B, Cseh J et al. (2011). Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Brit J Nutr 106: 383–389. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

