Biophotons Contribute to Retinal Dark Noise
- PMID: 27059222
- PMCID: PMC5563773
- DOI: 10.1007/s12264-016-0029-6
Biophotons Contribute to Retinal Dark Noise
Abstract
The discovery of dark noise in retinal photoreceptors resulted in a long-lasting controversy over its origin and the underlying mechanisms. Here, we used a novel ultra-weak biophoton imaging system (UBIS) to detect biophotonic activity (emission) under dark conditions in rat and bullfrog (Rana catesbeiana) retinas in vitro. We found a significant temperature-dependent increase in biophotonic activity that was completely blocked either by removing intracellular and extracellular Ca(2+) together or inhibiting phosphodiesterase 6. These findings suggest that the photon-like component of discrete dark noise may not be caused by a direct contribution of the thermal activation of rhodopsin, but rather by an indirect thermal induction of biophotonic activity, which then activates the retinal chromophore of rhodopsin. Therefore, this study suggests a possible solution regarding the thermal activation energy barrier for discrete dark noise, which has been debated for almost half a century.
Keywords: Biophoton; Biophoton imaging; Ca2+; Phosphodiesterase 6; Rat and bullfrog retinas; Retinal dark noise.
Figures
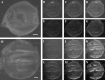
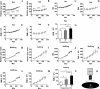
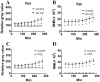
Similar articles
-
Rejection of the biophoton hypothesis on the origin of photoreceptor dark noise.J Gen Physiol. 2019 Jul 1;151(7):887-897. doi: 10.1085/jgp.201812317. Epub 2019 Apr 16. J Gen Physiol. 2019. PMID: 30992369 Free PMC article.
-
The limit of photoreceptor sensitivity: molecular mechanisms of dark noise in retinal cones.J Gen Physiol. 2005 Jun;125(6):641-60. doi: 10.1085/jgp.200509277. J Gen Physiol. 2005. PMID: 15928405 Free PMC article.
-
The Physical Mechanism for Retinal Discrete Dark Noise: Thermal Activation or Cellular Ultraweak Photon Emission?PLoS One. 2016 Mar 7;11(3):e0148336. doi: 10.1371/journal.pone.0148336. eCollection 2016. PLoS One. 2016. PMID: 26950936 Free PMC article.
-
Light-regulated enzymes of vertebrate retinal rods.Adv Cyclic Nucleotide Res. 1979;11:265-301. Adv Cyclic Nucleotide Res. 1979. PMID: 227247 Review. No abstract available.
-
Physical aspects of biophotons.Experientia. 1988 Jul 15;44(7):576-85. doi: 10.1007/BF01953305. Experientia. 1988. PMID: 3294033 Review.
Cited by
-
Rejection of the biophoton hypothesis on the origin of photoreceptor dark noise.J Gen Physiol. 2019 Jul 1;151(7):887-897. doi: 10.1085/jgp.201812317. Epub 2019 Apr 16. J Gen Physiol. 2019. PMID: 30992369 Free PMC article.
-
Biophotonic Activity and Transmission Mediated by Mutual Actions of Neurotransmitters are Involved in the Origin and Altered States of Consciousness.Neurosci Bull. 2018 Jun;34(3):534-538. doi: 10.1007/s12264-018-0215-9. Epub 2018 Mar 5. Neurosci Bull. 2018. PMID: 29508252 Free PMC article. No abstract available.
-
Human high intelligence is involved in spectral redshift of biophotonic activities in the brain.Proc Natl Acad Sci U S A. 2016 Aug 2;113(31):8753-8. doi: 10.1073/pnas.1604855113. Epub 2016 Jul 18. Proc Natl Acad Sci U S A. 2016. PMID: 27432962 Free PMC article.
-
The mechanism of photon-like dark noise in rod photoreceptors.J Gen Physiol. 2019 Jul 1;151(7):875-877. doi: 10.1085/jgp.201912376. Epub 2019 Jun 6. J Gen Physiol. 2019. PMID: 31171571 Free PMC article.
References
-
- Liu J, Liu MY, Nguyen JB, Bhagat A, Mooney V, Yan EC. Thermal decay of rhodopsin: role of hydrogen bonds in thermal isomerization of 11-cis retinal in the binding site and hydrolysis of protonated Schiff base. Journal of the American Chemical Society. 2009;131:8750–8751. doi: 10.1021/ja903154u. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

