A Preliminary Assessment of Rotavirus Vaccine Effectiveness in Zambia
- PMID: 27059353
- PMCID: PMC11976676
- DOI: 10.1093/cid/civ1206
A Preliminary Assessment of Rotavirus Vaccine Effectiveness in Zambia
Abstract
Background: Diarrhea is the third leading cause of child death in Zambia. Up to one-third of diarrhea cases resulting in hospitalization and/or death are caused by vaccine-preventable rotavirus. In January 2012, Zambia initiated a pilot introduction of the Rotarix live, oral rotavirus vaccine in all public health facilities in Lusaka Province.
Methods: Between July 2012 and October 2013, we conducted a case-control study at 6 public sector sites to estimate rotavirus vaccine effectiveness (VE) in age-eligible children presenting with diarrhea. We computed the odds of having received at least 1 dose of Rotarix among children whose stool was positive for rotavirus antigen (cases) and children whose stool was negative (controls). We adjusted the resulting odds ratio (OR) for patient age, calendar month of presentation, and clinical site, and expressed VE as (1 - adjusted OR) × 100.
Results: A total of 91 rotavirus-positive cases and 298 rotavirus-negative controls who had under-5 card-confirmed vaccination status and were ≥6 months of age were included in the case-control analysis. Among rotavirus-positive children who were age-eligible to be vaccinated, 20% were hospitalized. Against rotavirus diarrhea of all severity, the adjusted 2-dose VE was 26% (95% confidence interval [CI], -30% to 58%) among children ≥6 months of age. VE against hospitalized children ≥6 months of age was 56% (95% CI, -34% to 86%).
Conclusions: We observed a higher point estimate for VE against increased severity of illness compared with milder disease, but were not powered to detect a low level of VE against milder disease.
Keywords: Zambia; immunization; rotavirus; rotavirus vaccine; vaccine effectiveness.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail journals.permissions@oup.com.
Conflict of interest statement
Conflict of Interest Information for All Authors:
The authors declare no conflicts of interest in the work published here.
Figures
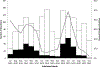
 Rotavirus Positive
Rotavirus Positive  Rotavirus Negative
Rotavirus Negative  % Rotavirus Positive
% Rotavirus Positive
 Rota Dose 1
Rota Dose 1  Rota Dose 2
Rota Dose 2  Penta Dose 1
Penta Dose 1  Penta Dose 2
Penta Dose 2References
-
- Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet (London, England) 2012; 379(9832): 2151–61. - PubMed
-
- UNICEF/WHO. Diarrhoea: Why cildren are still dying and what can be done. Geneva, Switzerland: WHO Press, 2009.
-
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. The Lancet Infectious diseases 2012; 12(2): 136–41. - PubMed
-
- ZMOH. 2008 Annual Health Statistical Bulletin. In: Health ZMo. Lusaka, 2009.
-
- Steele AD, Kasolo FC, Bos P, Peenze I, Oshitani H, Mpabalwani E. Characterization of VP6 subgroup, VP7 and VP4 genotype of rotavirus strains in Lusaka, Zambia. Annals of tropical paediatrics 1998; 18(2): 111–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

