Impact of Rotavirus Vaccination on Diarrheal Hospitalizations in Children Aged <5 Years in Lusaka, Zambia
- PMID: 27059354
- PMCID: PMC11977292
- DOI: 10.1093/cid/civ1027
Impact of Rotavirus Vaccination on Diarrheal Hospitalizations in Children Aged <5 Years in Lusaka, Zambia
Abstract
Background: Monovalent rotavirus vaccine was introduced in the routine public health immunization program in Lusaka, Zambia, in January 2012 and was rolled out countrywide in November 2013. We examined the effect of rotavirus vaccination on hospitalization for all-cause acute gastroenteritis (AGE) and rotavirus-specific AGE at a large referral hospital in Lusaka.
Methods: Data were derived from ongoing hospital-based AGE surveillance from January 2009 to December 2014. Pre-rotavirus vaccine introduction (2009-2011) and post-rotavirus vaccine introduction (2013-2014) periods were compared for annual changes in hospitalizations for AGE and rotavirus; 2012 was excluded as a transition year. Hospital administrative discharge data were used to compare trends in all-cause diarrhea discharges and in-hospital diarrhea deaths captured by HIMS pre- and post-rotavirus vaccine introduction.
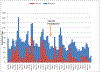
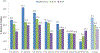
Results: Between January 2009 and December 2014, 5937 children <5 years of age presenting with AGE had their stools collected and tested for rotavirus by enzyme immunoassay. The rotavirus positivity rate declined from 40.1% (449/1121) in prevaccine years to 30.2% (250/828;P< .001) in 2013 and 24.7% (157/635;P< .001) in 2014. The greatest reduction was noted in infants, with the rotavirus positivity rate in this age group declining from 40.9% in prevaccine years to 34.0% (P= .009) in 2013 and 26.2% (P< .001) in 2014. Following rotavirus vaccine introduction, seasonal peaks of rotavirus and all-cause AGE were dwarfed. From HIMS data, compared to the prevaccine era, reductions of 18%-29% in all-cause diarrhea hospitalizations and 27%-33% in-hospital diarrhea deaths among children <1 year of age were observed in 2013 and 2014.
Conclusions: We observed a significant reduction in AGE-associated in-hospital morbidity and mortality following rotavirus vaccine introduction. The greatest reduction was seen in infants <1 year who accounted for 84.4% of rotavirus hospitalizations prior to vaccine introduction.
Keywords: acute gastroenteritis; disease burden; impact; rotavirus vaccine.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail journals.permissions@oup.com.
Figures
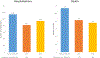
References
-
- Patel M, Steele D, Gentsch JR, Wecker J, Glass RI, Parashar UD. Real-World impact of rotavirus vaccination. PIDJ 2011;30 (Suppl):S1–S4 - PubMed
-
- O’Ryan M, Giaquinto C, Benninghoff B. Human rotavirus vaccine (Rotarix): Focus on effectiveness and impact 6 years after first introduction in Africa. Expert Rev. Vaccines 2015;14(8):1099–1112 - PubMed
-
- Cortese MM, Tate JE, Simonsen L, Edelman L, Parasher UD. Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatric Infect Dis J. 2010. Jun;29(6):489–94 - PubMed
-
- Curns AT, Steiner CA, Barrett M, Hunter K, Wilson E, Parashar UD. Reduction in acute gastroenteritis hospitalizations among US children after introduction of rotavirus vaccine: analysis of hospital discharge data from 18 US states. J Infect Dis. 2010. Jun 1;201(11):1617–24 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

