Temporal Association of Rotavirus Vaccine Introduction and Reduction in All-Cause Childhood Diarrheal Hospitalizations in South Africa
- PMID: 27059355
- PMCID: PMC11345717
- DOI: 10.1093/cid/civ1204
Temporal Association of Rotavirus Vaccine Introduction and Reduction in All-Cause Childhood Diarrheal Hospitalizations in South Africa
Abstract
Background: The public health impact of rotavirus vaccination in African settings with a high human immunodeficiency virus (HIV) infection prevalence is yet to be established. We evaluated trends in all-cause diarrheal hospitalizations in Soweto, Johannesburg, before and after the introduction of rotavirus vaccine into South Africa's national immunization program in August 2009.
Methods: Hospitalizations in children <5 years of age with a diagnosis of diarrhea, defined byInternational Classification of Diseases, Tenth Revisioncodes A00-A05, A06.0-A06.3, A06.9, A07.0-A07.2, A07.9, and A08-A09, were identified at the Chris Hani Baragwanath Academic Hospital from 1 January 2006 to 31 December 2014. The median annual prevaccine (2006-2008) hospitalization incidence was compared to that of the vaccine era (2010-2014), and stratified by age group and HIV infection status.
Results: Incidence reductions (per 1000 population) were greatest in children aged <12 months: 54.4 in the prevaccine era vs 30.0, 23.6, 20.0, 18.8, and 18.9 in the postvaccine years 2010-2014, respectively (a 44.9%-65.4% reduction). Lower incidence reductions (39.8%-49.4%) were observed among children aged 12-24 months from the second year post-vaccine introduction onward. Reductions were observed in both HIV-infected and HIV-uninfected children. There was a change in the seasonal pattern of diarrheal hospitalizations post-vaccine introduction, with flattening of the autumn-winter peaks seen in the prevaccine years.
Conclusions: An accelerated and sustained decline in all-cause diarrheal hospitalizations, temporally associated with rotavirus vaccine introduction, was observed in children <2 years of age. However, the impact of other interventions such as improved sanitation and changes in HIV management cannot be discounted.
Keywords: HIV; children; diarrhea; rotavirus vaccine.
Published by Oxford University Press for the Infectious Diseases Society of America 2016. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Conflict of interest statement
Figures
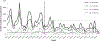


References
-
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008. estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:136–41. - PubMed
-
- Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354:11–22. - PubMed
-
- Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354:23–33. - PubMed
-
- Vesikari T, Karvonen A, Prymula R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet 2007; 370:1757–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

