Impact and Effectiveness of Monovalent Rotavirus Vaccine Against Severe Rotavirus Diarrhea in Ghana
- PMID: 27059357
- PMCID: PMC7962386
- DOI: 10.1093/cid/ciw014
Impact and Effectiveness of Monovalent Rotavirus Vaccine Against Severe Rotavirus Diarrhea in Ghana
Abstract
Background: Ghana was among the first African nations to introduce monovalent rotavirus vaccine (RV1) into its childhood immunization schedule in April 2012. We aimed to assess the impact of vaccine introduction on rotavirus and acute gastroenteritis (AGE) hospitalizations and to estimate vaccine effectiveness (VE).
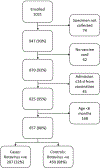
Methods: Using data from 2 teaching hospitals, monthly AGE and rotavirus admissions by age were examined 40 months before and 31 months after RV1 introduction using interrupted time-series analyses. From January 2013, we enrolled children <2 years of age who were eligible for RV1 from a total of 7 sentinel sites across the country. To estimate VE, we fit unconditional logistic regression models to calculate odds ratios of vaccination by rotavirus case-patient status, controlling for potential confounders.
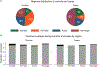
Results: Vaccine coverage ranged from 95% to 100% for dose 1 and 93% to 100% for dose 2. In the first 3 years after vaccine introduction, the percentage of hospital admissions positive for rotavirus fell from 48% in the prevaccine period to 28% (49% adjusted rate reduction; 95% confidence interval [CI], 32%-63%) postvaccination among <5-year-olds. With high vaccine coverage, it was not possible to arrive at robust VE estimates; any-dose VE against rotavirus hospitalization was estimated at 60% (95% CI, -2% to 84%;P= .056).
Conclusions: Results from the first 3 years following RV1 introduction suggest substantial reductions of pediatric diarrheal disease as a result of vaccination. Our VE estimate is consistent with the observed rotavirus decrease and with efficacy estimates from elsewhere in sub-Saharan Africa.
Keywords: Ghana; case-control; rotavirus; surveillance; vaccine effectiveness.
Published by Oxford University Press for the Infectious Diseases Society of America 2016. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Conflict of interest statement
Figures

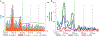
References
-
- World Health Organization. Immunization, vaccines and biologicals. Geneva, Switzerland: WHO, 2010.
-
- Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:606–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

