Population Impact and Effectiveness of Monovalent Rotavirus Vaccination in Urban Malawian Children 3 Years After Vaccine Introduction: Ecological and Case-Control Analyses
- PMID: 27059359
- PMCID: PMC4825885
- DOI: 10.1093/cid/civ1183
Population Impact and Effectiveness of Monovalent Rotavirus Vaccination in Urban Malawian Children 3 Years After Vaccine Introduction: Ecological and Case-Control Analyses
Abstract
Background: Rotavirus vaccines have been introduced in many low-income African countries including Malawi in 2012. Despite early evidence of vaccine impact, determining persistence of protection beyond infancy, the utility of the vaccine against specific rotavirus genotypes, and effectiveness in vulnerable subgroups is important.
Methods: We compared rotavirus prevalence in diarrheal stool and hospitalization incidence before and following rotavirus vaccine introduction in Malawi. Using case-control analysis, we derived vaccine effectiveness (VE) in the second year of life and for human immunodeficiency virus (HIV)-exposed and stunted children.
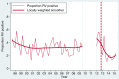
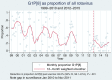
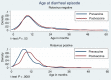
Results: Rotavirus prevalence declined concurrent with increasing vaccine coverage, and in 2015 was 24% compared with prevaccine mean baseline in 1997-2011 of 32%. Since vaccine introduction, population rotavirus hospitalization incidence declined in infants by 54.2% (95% confidence interval [CI], 32.8-68.8), but did not fall in older children. Comparing 241 rotavirus cases with 692 test-negative controls, VE was 70.6% (95% CI, 33.6%-87.0%) and 31.7% (95% CI, -140.6% to 80.6%) in the first and second year of life, respectively, whereas mean age of rotavirus cases increased from 9.3 to 11.8 months. Despite higher VE against G1P[8] than against other genotypes, no resurgence of nonvaccine genotypes has occurred. VE did not differ significantly by nutritional status (78.1% [95% CI, 5.6%-94.9%] in 257 well-nourished and 27.8% [95% CI, -99.5% to 73.9%] in 205 stunted children;P= .12), or by HIV exposure (60.5% [95% CI, 13.3%-82.0%] in 745 HIV-unexposed and 42.2% [95% CI, -106.9% to 83.8%] in 174 exposed children;P= .91).
Conclusions: Rotavirus vaccination in Malawi has resulted in reductions in disease burden in infants <12 months, but not in older children. Despite differences in genotype-specific VE, no genotype has emerged to suggest vaccine escape. VE was not demonstrably affected by HIV exposure or stunting.
Keywords: case-control; developing countries; population impact; rotavirus vaccine; vaccine effectiveness.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures


References
-
- Madhi SA, Cunliffe NA, Steele D et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 2010; 362:289–98. - PubMed
-
- World Health Organization, Strategic Advisory Group of Experts. Meeting of the immunization Strategic Advisory Group of Experts, April 2009—conclusions and recommendations. Wkly Epidemiol Rec 2009; 84:213–36.
-
- Program for AppropriateTechnologies in Health (PATH). Rotavirus vaccine access and delivery. 2014. Available at: http://sites.path.org/rotavirusvaccine/country-introduction-maps-and-spr.... Accessed 3 January 2016.
-
- Groome MJ, Page N, Cortese MM et al. Effectiveness of monovalent human rotavirus vaccine against admission to hospital for acute rotavirus diarrhoea in South African children: a case-control study. Lancet Infect Dis 2014; 14:1096–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

