Mitochondrial Transfer via Tunneling Nanotubes is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS
- PMID: 27059413
- PMCID: PMC4982045
- DOI: 10.1002/stem.2372
Mitochondrial Transfer via Tunneling Nanotubes is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS
Abstract
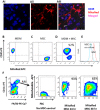
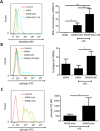
Mesenchymal stromal cells (MSC) have been reported to improve bacterial clearance in preclinical models of Acute Respiratory Distress Syndrome (ARDS) and sepsis. The mechanism of this effect is not fully elucidated yet. The primary objective of this study was to investigate the hypothesis that the antimicrobial effect of MSC in vivo depends on their modulation of macrophage phagocytic activity which occurs through mitochondrial transfer. We established that selective depletion of alveolar macrophages (AM) with intranasal (IN) administration of liposomal clodronate resulted in complete abrogation of MSC antimicrobial effect in the in vivo model of Escherichia coli pneumonia. Furthermore, we showed that MSC administration was associated with enhanced AM phagocytosis in vivo. We showed that direct coculture of MSC with monocyte-derived macrophages enhanced their phagocytic capacity. By fluorescent imaging and flow cytometry we demonstrated extensive mitochondrial transfer from MSC to macrophages which occurred at least partially through tunneling nanotubes (TNT)-like structures. We also detected that lung macrophages readily acquire MSC mitochondria in vivo, and macrophages which are positive for MSC mitochondria display more pronounced phagocytic activity. Finally, partial inhibition of mitochondrial transfer through blockage of TNT formation by MSC resulted in failure to improve macrophage bioenergetics and complete abrogation of the MSC effect on macrophage phagocytosis in vitro and the antimicrobial effect of MSC in vivo. Collectively, this work for the first time demonstrates that mitochondrial transfer from MSC to innate immune cells leads to enhancement in phagocytic activity and reveals an important novel mechanism for the antimicrobial effect of MSC in ARDS. Stem Cells 2016;34:2210-2223.
Keywords: ARDS; Macrophages; Mesenchymal stem cells; Mitochondrial transfer; Phagocytosis.
© 2016 The Authors STEM CELLS published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures





References
-
- Ranieri V, Rubenfeld G, Thompson B et al. Acute respiratory distress syndrome: The Berlin definition. JAMA 2012;307: 2526–2533. - PubMed
-
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349. - PubMed
-
- Alhadlaq A, Mao JJ. Mesenchymal stem cells: Isolation and therapeutics. Stem Cells Dev 2004;13:436–448. - PubMed
-
- Hoogduijn MJ, Crop MJ, Peeters AMA et al. Human heart, spleen, and perirenal fat‐derived mesenchymal stem cells have immunomodulatory capacities. Stem Cells Dev 2007;16:597–604. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

