Nicorandil attenuates carotid intimal hyperplasia after balloon catheter injury in diabetic rats
- PMID: 27059601
- PMCID: PMC4826484
- DOI: 10.1186/s12933-016-0377-6
Nicorandil attenuates carotid intimal hyperplasia after balloon catheter injury in diabetic rats
Abstract
Background: Diabetic patients suffer from undesired intimal hyperplasia after angioplasty. Nicorandil has a trend to reduce the rate of target lesion revascularization. However, whether nicorandil inhibits intimal hyperplasia and the possible mechanisms underlying it remain to be determined. We aimed at assessing the effect of nicorandil on intimal hyperplasia in diabetic rats.
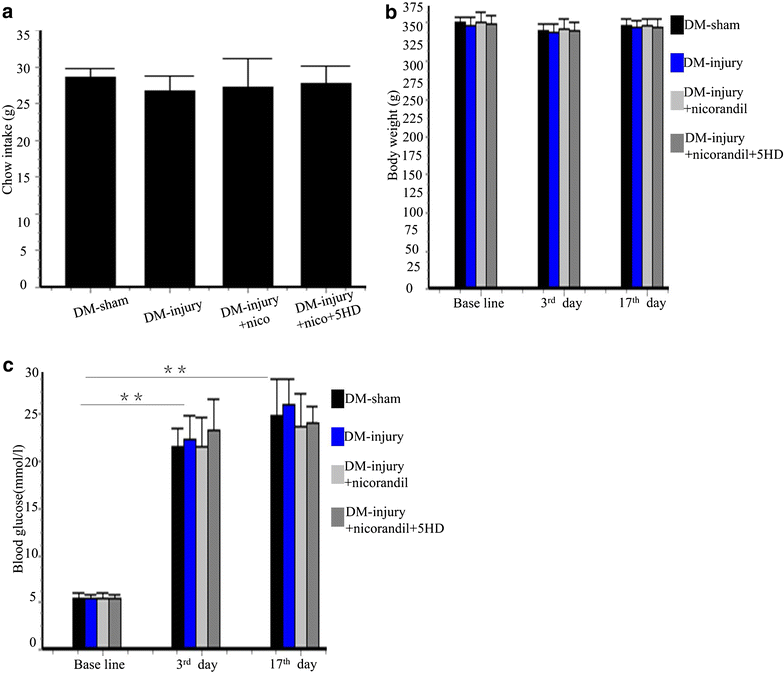
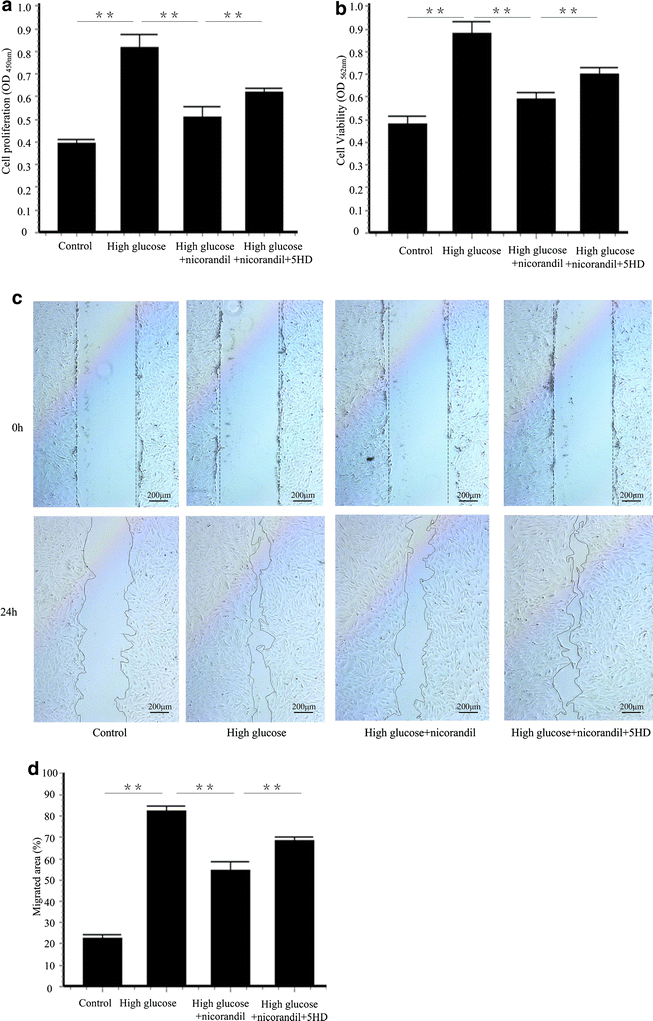
Methods: After intraperitoneal injection of streptozotocin (STZ, 50 mg/kg), balloon injury model was established in carotid arteries of diabetic rats. Rats were randomized to vehicle, nicorandil (15 mg/kg/day) or 5-hydroxydecanoate (5-HD, 10 mg/kg/day), a mitochondrial ATP-sensitive potassium channel (mitoKATP channel)-selective antagonist. Perivascular delivery of εPKC siRNA was conducted to determine the role of εPKC pathway in intimal hyperplasia. In hyperglycemia environment (25 mM glucose), primary culture of vascular smooth muscle cells (VSMCs) were treated with nicorandil or 5-HD. Cell proliferation and cell migration were analyzed.
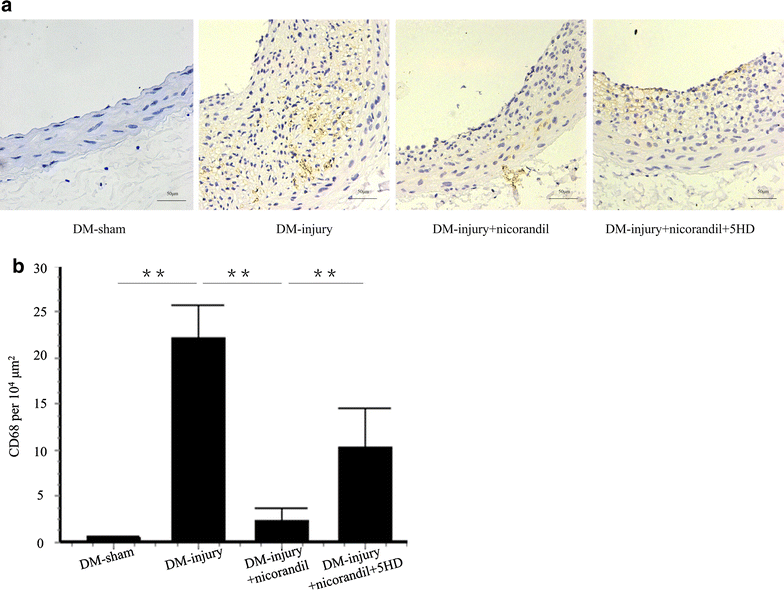
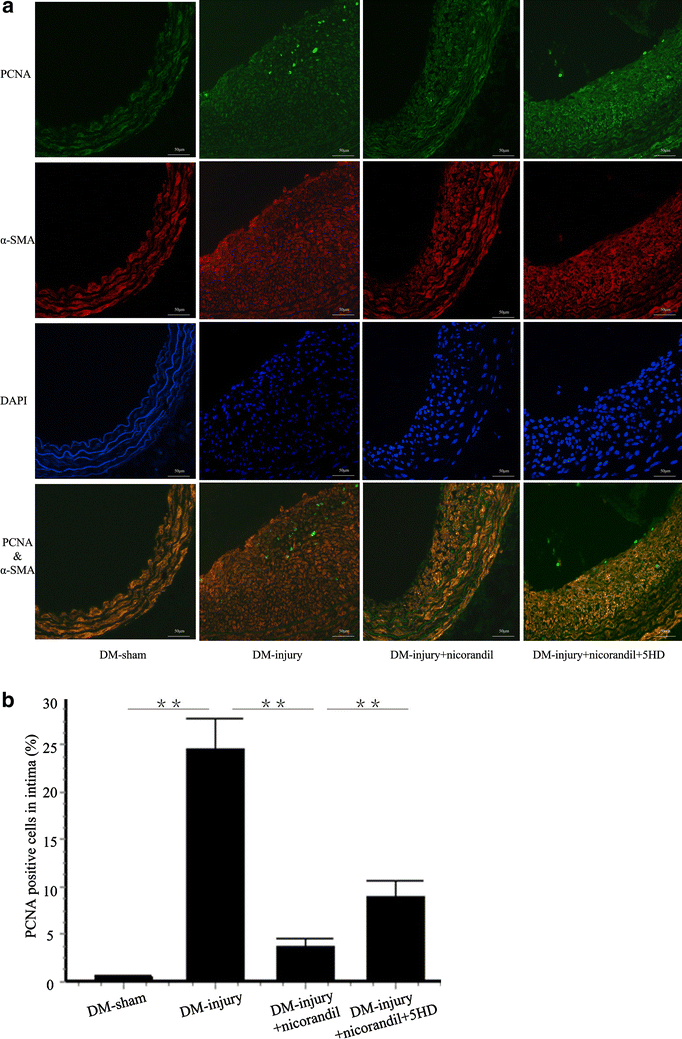
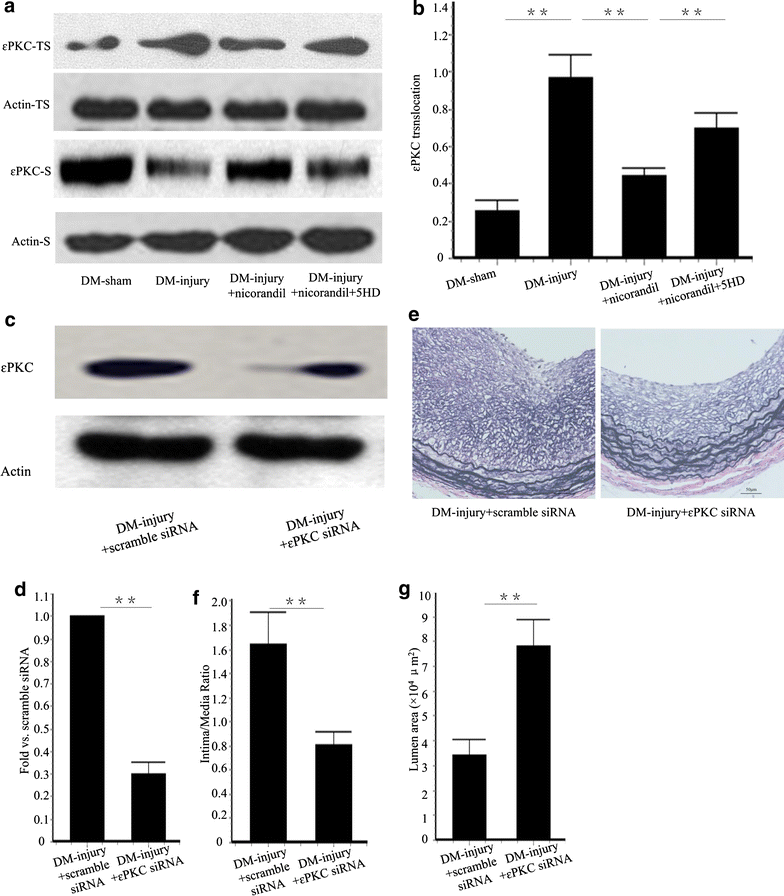
Results: Intimal hyperplasia significantly increased 14 days after balloon injury in diabetic rats (p < 0.01). Nicorandil inhibited intima development, reduced inflammation and prevented cell proliferation in balloon-injured arteries (p < 0.01). The protective effects of nicorandil were reversed by 5-HD (p < 0.05). εPKC was activated in balloon-injured arteries (p < 0.01). Nicorandil inhibited εPKC activation by opening mitoKATP channel. Perivascular delivery of εPKC siRNA inhibited intimal hyperplasia, inflammation and cell proliferation (p < 0.01). High glucose-induced VSMCs proliferation and migration were inhibited by nicorandil. εPKC activation induced by high glucose was also inhibited by nicorandil and that is partially reversed by 5-HD. εPKC knockdown prevented VSMCs proliferation and migration (p < 0.01).
Conclusions: Our study demonstrates that nicorandil inhibits intimal hyperplasia in balloon-injured arteries in diabetic rats. Nicorandil also prevents VSMCs proliferation and migration induced by high glucose. The beneficial effect of nicorandil is conducted via opening mitoKATP channel and inhibiting εPKC activation.
Keywords: ATP-sensitive potassium channel; Diabetes mellitus; Intimal hyperplasia; Nicorandil; Protein kinase C.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

