α-Synuclein-induced myelination deficit defines a novel interventional target for multiple system atrophy
- PMID: 27059609
- PMCID: PMC4912450
- DOI: 10.1007/s00401-016-1572-y
α-Synuclein-induced myelination deficit defines a novel interventional target for multiple system atrophy
Abstract
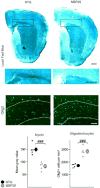
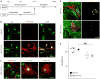
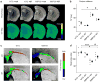
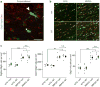
Multiple system atrophy (MSA) is a rare atypical parkinsonian disorder characterized by a rapidly progressing clinical course and at present without any efficient therapy. Neuropathologically, myelin loss and neurodegeneration are associated with α-synuclein accumulation in oligodendrocytes, but underlying pathomechanisms are poorly understood. Here, we analyzed the impact of oligodendrocytic α-synuclein on the formation of myelin sheaths to define a potential interventional target for MSA. Post-mortem analyses of MSA patients and controls were performed to quantify myelin and oligodendrocyte numbers. As pre-clinical models, we used transgenic MSA mice, a myelinating stem cell-derived oligodendrocyte-neuron co-culture, and primary oligodendrocytes to determine functional consequences of oligodendrocytic α-synuclein overexpression on myelination. We detected myelin loss accompanied by preserved or even increased numbers of oligodendrocytes in post-mortem MSA brains or transgenic mouse forebrains, respectively, indicating an oligodendrocytic dysfunction in myelin formation. Corroborating this observation, overexpression of α-synuclein in primary and stem cell-derived oligodendrocytes severely impaired myelin formation, defining a novel α-synuclein-linked pathomechanism in MSA. We used the pro-myelinating activity of the muscarinic acetylcholine receptor antagonist benztropine to analyze the reversibility of the myelination deficit. Transcriptome profiling of primary pre-myelinating oligodendrocytes demonstrated that benztropine readjusts myelination-related processes such as cholesterol and membrane biogenesis, being compromised by oligodendrocytic α-synuclein. Additionally, benztropine restored the α-synuclein-induced myelination deficit of stem cell-derived oligodendrocytes. Strikingly, benztropine also ameliorated the myelin deficit in transgenic MSA mice, resulting in a prevention of neuronal cell loss. In conclusion, this study defines the α-synuclein-induced myelination deficit as a novel and crucial pathomechanism in MSA. Importantly, the reversible nature of this oligodendrocytic dysfunction opens a novel avenue for an intervention in MSA.
Keywords: Multiple system atrophy; Myelin; Oligodendrocyte progenitor cells; Oligodendrocytes; α-Synuclein.
Conflict of interest statement
Figures





Similar articles
-
Endogenous oligodendroglial alpha-synuclein and TPPP/p25α orchestrate alpha-synuclein pathology in experimental multiple system atrophy models.Acta Neuropathol. 2019 Sep;138(3):415-441. doi: 10.1007/s00401-019-02014-y. Epub 2019 Apr 22. Acta Neuropathol. 2019. PMID: 31011860 Free PMC article.
-
α-Synuclein impairs oligodendrocyte progenitor maturation in multiple system atrophy.Neurobiol Aging. 2014 Oct;35(10):2357-68. doi: 10.1016/j.neurobiolaging.2014.02.028. Epub 2014 Mar 4. Neurobiol Aging. 2014. PMID: 24698767 Free PMC article.
-
A rapidly progressive multiple system atrophy-cerebellar variant model presenting marked glial reactions with inflammation and spreading of α-synuclein oligomers and phosphorylated α-synuclein aggregates.Brain Behav Immun. 2024 Oct;121:122-141. doi: 10.1016/j.bbi.2024.07.004. Epub 2024 Jul 8. Brain Behav Immun. 2024. PMID: 38986725
-
Novel therapeutic approaches in multiple system atrophy.Clin Auton Res. 2015 Feb;25(1):37-45. doi: 10.1007/s10286-014-0249-7. Epub 2014 Jun 14. Clin Auton Res. 2015. PMID: 24928797 Free PMC article. Review.
-
Insights into the pathogenesis of multiple system atrophy: focus on glial cytoplasmic inclusions.Transl Neurodegener. 2020 Feb 17;9:7. doi: 10.1186/s40035-020-0185-5. eCollection 2020. Transl Neurodegener. 2020. PMID: 32095235 Free PMC article. Review.
Cited by
-
CSF1R-Mediated Myeloid Cell Depletion Prolongs Lifespan But Aggravates Distinct Motor Symptoms in a Model of Multiple System Atrophy.J Neurosci. 2022 Oct 5;42(40):7673-7688. doi: 10.1523/JNEUROSCI.0417-22.2022. Epub 2022 Sep 6. J Neurosci. 2022. PMID: 36333098 Free PMC article.
-
Multiple system atrophy: α-Synuclein strains at the neuron-oligodendrocyte crossroad.Mol Neurodegener. 2022 Nov 26;17(1):77. doi: 10.1186/s13024-022-00579-z. Mol Neurodegener. 2022. PMID: 36435784 Free PMC article. Review.
-
Neurons and Glia Interplay in α-Synucleinopathies.Int J Mol Sci. 2021 May 8;22(9):4994. doi: 10.3390/ijms22094994. Int J Mol Sci. 2021. PMID: 34066733 Free PMC article. Review.
-
Current Symptomatic and Disease-Modifying Treatments in Multiple System Atrophy.Int J Mol Sci. 2020 Apr 16;21(8):2775. doi: 10.3390/ijms21082775. Int J Mol Sci. 2020. PMID: 32316335 Free PMC article. Review.
-
Higher levels of myelin phospholipids in brains of neuronal α-Synuclein transgenic mice precede myelin loss.Acta Neuropathol Commun. 2017 May 8;5(1):37. doi: 10.1186/s40478-017-0439-3. Acta Neuropathol Commun. 2017. PMID: 28482862 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

