The therapeutic potential of mTOR inhibitors in breast cancer
- PMID: 27059645
- PMCID: PMC5061784
- DOI: 10.1111/bcp.12958
The therapeutic potential of mTOR inhibitors in breast cancer
Abstract
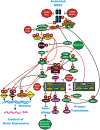
Rapamycin and modified rapamycins (rapalogs) have been used to prevent allograft rejection after organ transplant for over 15 years. The mechanistic target of rapamycin (mTOR) has been determined to be a key component of the mTORC1 complex which consists of the serine/threonine kinase TOR and at least five other proteins which are involved in regulating its activity. Some of the best characterized substrates of mTORC1 are proteins which are key kinases involved in the regulation of cell growth (e.g., p70S6K) and protein translation (e.g., 4E-BP1). These proteins may in some cases serve as indicators to sensitivity to rapamycin-related therapies. Dysregulation of mTORC1 activity frequently occurs due to mutations at, or amplifications of, upstream growth factor receptors (e.g., human epidermal growth factor receptor-2, HER2) as well as kinases (e.g., PI3K) and phosphatases (e.g., PTEN) critical in the regulation of cell growth. More recently, it has been shown that certain rapalogs may enhance the effectiveness of hormonal-based therapies for breast cancer patients who have become resistant to endocrine therapy. The combined treatment of certain rapalogs (e.g., everolimus) and aromatase inhibitors (e.g., exemestane) has been approved by the United States Food and Drug Administration (US FDA) and other drug regulatory agencies to treat estrogen receptor positive (ER+) breast cancer patients who have become resistant to hormonal-based therapies and have progressed. This review will summarize recent basic and clinical research in the area and evaluate potential novel therapeutic approaches.
Keywords: drug resistance; endocrine resistance; everolimus; exemestane; metastasis; rapamycin.
© 2016 The British Pharmacological Society.
Figures


References
-
- American Cancer Society , http://www.cancer.org/acs/groups/content/@research/documents/document/ac... (last accessed 15 December 2015).
-
- Bostner J, Karlsson E, Eding CB, Perez‐Tenorio G, Franzén H, Konstantinell A, et al. S6 kinase signaling: tamoxifen response and prognostic indication in two breast cancer cohorts. Endocr Relat Cancer 2015; 22: 331–43. - PubMed
-
- Chan S, Scheulen ME, Johnston S, Mross K, Cardoso F, Dittrich C, et al. Phase II study of temsirolimus (CCI‐779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol 2005; 23: 5314–22. - PubMed
-
- Dancey JE, Curiel R, Purvis J. Evaluating temsirolimus activity in multiple tumors: a review of clinical trials. Semin Oncol 2009; 36 (Suppl 3): S46–58. - PubMed
-
- Buckner JC, Forouzesh B, Erlichman C, Hidalgo M, Boni JP, Dukart G, et al. Phase I, pharmacokinetic study of temsirolimus administered orally to patients with advanced cancer. Invest New Drugs 2010; 28: 334–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

