Checkpoints for Autoreactive B Cells in the Peripheral Blood of Lupus Patients Assessed by Flow Cytometry
- PMID: 27059652
- PMCID: PMC5523861
- DOI: 10.1002/art.39710
Checkpoints for Autoreactive B Cells in the Peripheral Blood of Lupus Patients Assessed by Flow Cytometry
Abstract
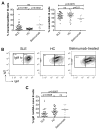
Objective: Antinuclear antibodies (ANAs) are diagnostic in several autoimmune disorders, yet the failure to achieve B cell tolerance in these diseases is still poorly understood. Although secreted ANAs detected by an indirect immunofluorescence assay are the gold standard for autoreactivity, there has been no convenient assay with which to measure the frequency of circulating B cells that recognize nuclear antigens (ANA+ B cells) in patients. The aim of this study was to generate an assay to easily identify these B cells and to examine its utility in a study of autoreactive B cells in systemic lupus erythematosus (SLE).
Methods: We developed and validated a novel flow cytometry-based assay that identifies ANA+ B cells using biotinylated nuclear extracts, and utilized it to examine B cell tolerance checkpoints in peripheral blood mononuclear cells obtained from SLE patients and healthy controls.
Results: We observed progressive selection against ANA+ B cells as they matured from transitional to naive to CD27+IgD- and CD27+IgD+ memory cells in both healthy subjects and SLE patients; however, ANA+ naive B cells in SLE patients were not anergized to the same extent as in healthy individuals. We also showed that anergy induction is restored in SLE patients treated with belimumab, an inhibitor of BAFF.
Conclusion: This assay will enable studies of large populations to identify potential genetic or environmental factors affecting B cell tolerance checkpoints in healthy subjects and patients with autoimmune disease and permit monitoring of the B cell response to therapeutic interventions.
© 2016, American College of Rheumatology.
Figures


References
-
- Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–7. - PubMed
-
- Yurasov S, Hammersen J, Tiller T, Tsuiji M, Wardemann H. B-cell tolerance checkpoints in healthy humans and patients with systemic lupus erythematosus. Ann N Y Acad Sci. 2005;1062:165–74. - PubMed
-
- Tiller T, Meffre E, Yurasov S, Tsuiji M, Nussenzweig MC, Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning [published erratum appears in J Immunol Methods 2008;334:142] J Immunol Methods. 2008;329:112–24. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

