Surge-Like Luteinising Hormone Secretion Induced by Retrochiasmatic Area NK3R Activation is Mediated Primarily by Arcuate Kisspeptin Neurones in the Ewe
- PMID: 27059932
- PMCID: PMC5157122
- DOI: 10.1111/jne.12393
Surge-Like Luteinising Hormone Secretion Induced by Retrochiasmatic Area NK3R Activation is Mediated Primarily by Arcuate Kisspeptin Neurones in the Ewe
Abstract
The neuropeptides neurokinin B (NKB) and kisspeptin are potent stimulators of gonadotrophin-releasing hormone (GnRH)/luteinsing hormone (LH) secretion and are essential for human fertility. We have recently demonstrated that selective activation of NKB receptors (NK3R) within the retrochiasmatic area (RCh) and the preoptic area (POA) triggers surge-like LH secretion in ovary-intact ewes, whereas blockade of RCh NK3R suppresses oestradiol-induced LH surges in ovariectomised ewes. Although these data suggest that NKB signalling within these regions of the hypothalamus mediates the positive-feedback effects of oestradiol on LH secretion, the pathway through which it stimulates GnRH/LH secretion remains unclear. We proposed that the action of NKB on RCh neurones drives the LH surge by stimulating kisspeptin-induced GnRH secretion. To test this hypothesis, we quantified the activation of the preoptic/hypothalamic populations of kisspeptin neurones in response to POA or RCh administration of senktide by dual-label immunohistochemical detection of kisspeptin and c-Fos (i.e. marker of neuronal activation). We then administered the NK3R agonist, senktide, into the RCh of ewes in the follicular phase of the oestrous cycle and conducted frequent blood sampling during intracerebroventricular infusion of the kisspeptin receptor antagonist Kp-271 or saline. Our results show that the surge-like secretion of LH induced by RCh senktide administration coincided with a dramatic increase in c-Fos expression within arcuate nucleus (ARC) kisspeptin neurones, and was completely blocked by Kp-271 infusion. We substantiate these data with evidence of direct projections of RCh neurones to ARC kisspeptin neurones. Thus, NKB-responsive neurones in the RCh act to stimulate GnRH secretion by inducing kisspeptin release from KNDy neurones.
Keywords: KNDy neurones; LH surge; NK3R; kisspeptin; retrochiasmatic area; tachykinins.
© 2016 British Society for Neuroendocrinology.
Figures
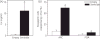
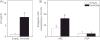





Similar articles
-
Neurokinin-3 receptor activation in the retrochiasmatic area is essential for the full pre-ovulatory luteinising hormone surge in ewes.J Neuroendocrinol. 2014 Nov;26(11):776-84. doi: 10.1111/jne.12180. J Neuroendocrinol. 2014. PMID: 25040132 Free PMC article.
-
Evidence that Neurokinin B Controls Basal Gonadotropin-Releasing Hormone Secretion but Is Not Critical for Estrogen-Positive Feedback in Sheep.Neuroendocrinology. 2015;101(2):161-74. doi: 10.1159/000377702. Epub 2015 Feb 12. Neuroendocrinology. 2015. PMID: 25677216
-
Prenatal testosterone excess decreases neurokinin 3 receptor immunoreactivity within the arcuate nucleus KNDy cell population.J Neuroendocrinol. 2015 Feb;27(2):100-10. doi: 10.1111/jne.12244. J Neuroendocrinol. 2015. PMID: 25496429 Free PMC article.
-
Kisspeptin and the preovulatory gonadotrophin-releasing hormone/luteinising hormone surge in the ewe: basic aspects and potential applications in the control of ovulation.J Neuroendocrinol. 2010 Jul;22(7):710-5. doi: 10.1111/j.1365-2826.2010.02022.x. Epub 2010 May 8. J Neuroendocrinol. 2010. PMID: 20456610 Review.
-
A role for neurokinin B in pulsatile GnRH secretion in the ewe.Neuroendocrinology. 2014;99(1):18-32. doi: 10.1159/000355285. Epub 2013 Oct 4. Neuroendocrinology. 2014. PMID: 24008670 Free PMC article. Review.
Cited by
-
Morphological and functional evidence for sexual dimorphism in neurokinin B signalling in the retrochiasmatic area of sheep.J Neuroendocrinol. 2020 Jul;32(7):e12877. doi: 10.1111/jne.12877. Epub 2020 Jun 22. J Neuroendocrinol. 2020. PMID: 32572994 Free PMC article.
-
Evidence That Endogenous Somatostatin Inhibits Episodic, but Not Surge, Secretion of LH in Female Sheep.Endocrinology. 2017 Jun 1;158(6):1827-1837. doi: 10.1210/en.2017-00075. Endocrinology. 2017. PMID: 28379327 Free PMC article.
-
Evidence That the LH Surge in Ewes Involves Both Neurokinin B-Dependent and -Independent Actions of Kisspeptin.Endocrinology. 2019 Dec 1;160(12):2990-3000. doi: 10.1210/en.2019-00597. Endocrinology. 2019. PMID: 31599937 Free PMC article.
-
Critical role of arcuate nucleus kisspeptin and Kiss1R in regulation of the ovine luteinizing hormone surge.J Neuroendocrinol. 2025 May;37(5):e70010. doi: 10.1111/jne.70010. Epub 2025 Mar 3. J Neuroendocrinol. 2025. PMID: 40033679
-
Arcuate and Preoptic Kisspeptin Neurons Exhibit Differential Projections to Hypothalamic Nuclei and Exert Opposite Postsynaptic Effects on Hypothalamic Paraventricular and Dorsomedial Nuclei in the Female Mouse.eNeuro. 2021 Aug 6;8(4):ENEURO.0093-21.2021. doi: 10.1523/ENEURO.0093-21.2021. Print 2021 Jul-Aug. eNeuro. 2021. PMID: 34281980 Free PMC article.
References
-
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. - PubMed
-
- Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41:354–358. - PMC - PubMed
-
- Guran T, Tolhurst G, Bereket A, Rocha N, Porter K, Turan S, Gribble FM, Kotan LD, Akcay T, Atay Z, Canan H, Serin A, O’Rahilly S, Reimann F, Semple RK, Topaloglu AK. Hypogonadotropic hypogonadism due to a novel missense mutation in the first extracellular loop of the neurokinin B receptor. J Clin Endocrinol Metab. 2009;94:3633–3639. - PMC - PubMed
-
- Topaloglu AK, Tello JA, Kotan LD, Ozbek MN, Yilmaz MB, Erdogan S, Gurbuz F, Temiz F, Millar RP, Yuksel B. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;366:629–635. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

