Extracellular Regulation of Bone Morphogenetic Protein Activity by the Microfibril Component Fibrillin-1
- PMID: 27059954
- PMCID: PMC4933460
- DOI: 10.1074/jbc.M115.704734
Extracellular Regulation of Bone Morphogenetic Protein Activity by the Microfibril Component Fibrillin-1
Abstract
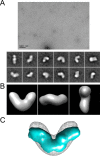
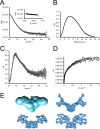
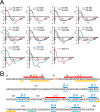
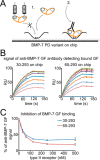
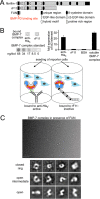
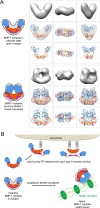
Since the discovery of bone morphogenetic proteins (BMPs) as pluripotent cytokines extractable from bone matrix, it has been speculated how targeting of BMPs to the extracellular matrix (ECM) modulates their bioavailability. Understanding these processes is crucial for elucidating pathomechanisms of connective tissue disorders characterized by ECM deficiency and growth factor dysregulation. Here, we provide evidence for a new BMP targeting and sequestration mechanism that is controlled by the ECM molecule fibrillin-1. We present the nanoscale structure of the BMP-7 prodomain-growth factor complex using electron microscopy, small angle x-ray scattering, and circular dichroism spectroscopy, showing that it assumes an open V-like structure when it is bioactive. However, upon binding to fibrillin-1, the BMP-7 complex is rendered into a closed ring shape, which also confers latency to the growth factor, as demonstrated by bioactivity measurements. BMP-7 prodomain variants were used to map the critical epitopes for prodomain-growth factor and prodomain-prodomain binding. Together, these data show that upon prodomain binding to fibrillin-1, the BMP-7 complex undergoes a conformational change, which denies access of BMP receptors to the growth factor.
Keywords: bone morphogenetic protein (BMP); electron microscopy (EM); extracellular matrix; fribrillin; growth factor; signal transduction; small-angle X-ray scattering (SAXS).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Shi Y., and Massagué J. (2003) Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113, 685–700 - PubMed
-
- Wu M. Y., and Hill C. S. (2009) Tgf-β superfamily signaling in embryonic development and homeostasis. Dev. Cell 16, 329–343 - PubMed
-
- Massagué J., and Chen Y. G. (2000) Controlling TGF-β signaling. Genes Dev. 14, 627–644 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

