The efficacy of continuous-flow cryo and cyclic compression therapy after hip fracture surgery on postoperative pain: design of a prospective, open-label, parallel, multicenter, randomized controlled, clinical trial
- PMID: 27059990
- PMCID: PMC4826534
- DOI: 10.1186/s12891-016-1000-4
The efficacy of continuous-flow cryo and cyclic compression therapy after hip fracture surgery on postoperative pain: design of a prospective, open-label, parallel, multicenter, randomized controlled, clinical trial
Abstract
Background: The number of hip fractures and resulting post-surgical outcome are a major public health concern and the incidence is expected to increase significantly. The acute recovery phase after hip fracture surgery in elder patients is often complicated by severe pain, high morphine consumption, perioperative blood loss with subsequent transfusion and delirium. Postoperative continuous-flow cryocompression therapy is suggested to minimize these complications and to attenuate the inflammatory reaction that the traumatic fracture and subsequent surgical trauma encompass. Based on a pilot study in patients undergoing total hip arthroplasty for osteoarthritis, it is anticipated that patients treated with continuous-flow cryocompression therapy will have less pain, less morphine consumption and lower decrease of postoperative hemoglobin levels. These factors are associated with a shorter hospital stay and better long-term (functional) outcome.
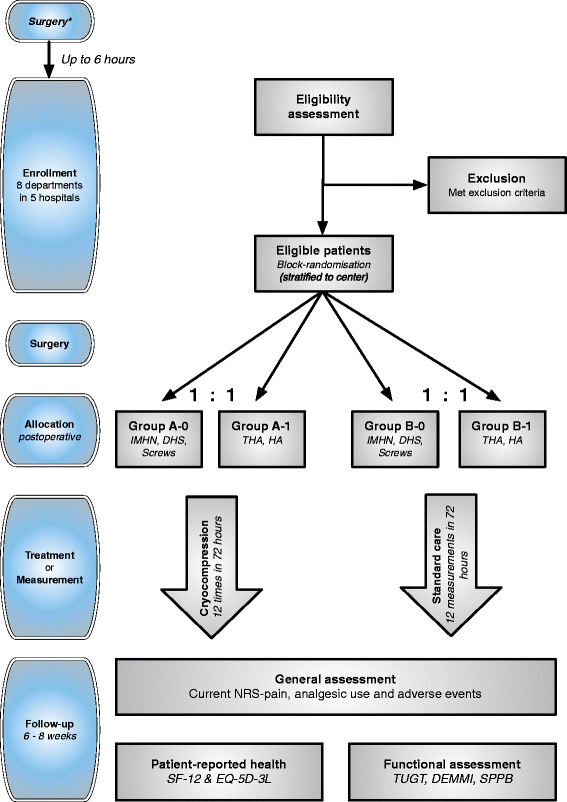
Methods/design: One hundred and sixty patients with an intra or extracapsular hip fracture scheduled for internal fixation (intramedullary hip nail, dynamic hip screw or cannulated screws) or prosthesis surgery (total hip or hemiarthroplasty) will be included in this prospective, open-label, parallel, multicenter, randomized controlled, clinical superiority trial. Patients will be allocated to two treatment arms: group 'A' will be treated with continuous-flow cryocompression therapy and compared to group 'B' that will receive standard care. Routine use of drains and/or compressive bandages is allowed in both groups. The primary objective of this study is to compare acute pain the first 72 h postoperative, measured with numeric rating scale for pain. Secondary objectives are: (non-) morphine analgesic use; adjusted postoperative hemoglobin level; transfusion incidence; incidence, duration and severity of delirium and use of psychotropic medication; length of stay; location and duration of rehabilitation; functional outcome; short-term patient-reported health outcome; general and cryotherapy related complications and feasibility.
Discussion: This is the first randomized controlled trial that will assess the analgesic efficiacy of continuous-flow cryocompression therapy in the acute recovery phase after hip fracture surgery.
Trial registration: www.trialregister.nl, NTR4152 (23(rd) of August 2013).
Keywords: Analgesia; Complications; Cryotherapy; Delirium; Functional outcome; Hemoglobin; Hip fracture; Induced hypothermia; Intermittent pneumatic compression device; Length of stay; Morphine; Opioid analgesics; Pain; Patient-reported outcome.
Figures

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

