Feeding Experimentation Device (FED): A flexible open-source device for measuring feeding behavior
- PMID: 27060385
- PMCID: PMC4884551
- DOI: 10.1016/j.jneumeth.2016.04.003
Feeding Experimentation Device (FED): A flexible open-source device for measuring feeding behavior
Abstract
Background: Measuring food intake in rodents is a conceptually simple yet labor-intensive and temporally-imprecise task. Most commonly, food is weighed manually, with an interval of hours or days between measurements. Commercial feeding monitors are excellent, but are costly and require specialized caging and equipment.
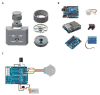
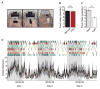
New method: We have developed the Feeding Experimentation Device (FED): a low-cost, open-source, home cage-compatible feeding system. FED utilizes an Arduino microcontroller and open-source software and hardware. FED dispenses a single food pellet into a food well where it is monitored by an infrared beam. When the mouse removes the pellet, FED logs the timestamp to a secure digital (SD) card and dispenses a new pellet into the well. Post-hoc analyses of pellet retrieval timestamps reveal high-resolution details about feeding behavior.
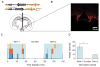
Results: FED is capable of accurately measuring food intake, identifying discrete trends during light and dark-cycle feeding. Additionally, we show the utility of FED for measuring increases in feeding resulting from optogenetic stimulation of agouti-related peptide neurons in the arcuate nucleus of the hypothalamus.
Comparison to existing methods: With a cost of ∼$350 per device, FED is >10× cheaper than commercially available feeding systems. FED is also self-contained, battery powered, and designed to be placed in standard colony rack cages, allowing for monitoring of true home cage feeding behavior. Moreover, FED is highly adaptable and can be synchronized with emerging techniques in neuroscience, such as optogenetics, as we demonstrate here.
Conclusions: FED allows for accurate, precise monitoring of feeding behavior in a home cage setting.
Keywords: Arduino; Feeding behavior; Food intake; Open-source.
Published by Elsevier B.V.
Figures

References
-
- Hoffman AM, Song J, Tuttle EM. ELOPTA: A novel microcontroller-based operant device. Behavior Research Methods. 2007;39(4):776–782. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

