Airway smooth muscle inflammation is regulated by microRNA-145 in COPD
- PMID: 27060571
- PMCID: PMC5082497
- DOI: 10.1002/1873-3468.12168
Airway smooth muscle inflammation is regulated by microRNA-145 in COPD
Abstract
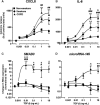
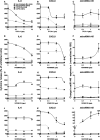
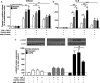
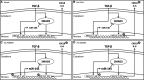
Chronic obstructive pulmonary disease (COPD) is a common, highly debilitating disease of the airways, primarily caused by smoking. Chronic inflammation and structural remodelling are key pathological features of this disease, in part caused by the aberrant function of airway smooth muscle (ASM) cells under the regulation of transforming growth factor (TGF)-β. miRNA are short, noncoding gene transcripts involved in the negative regulation of specific target genes, through their interactions with mRNA. Previous studies have proposed that mRNA-145 (miR-145) may interact with SMAD3, an important downstream signalling molecule of the TGF-β pathway. TGF-β was used to stimulate primary human ASM cells isolated from healthy nonsmokers, healthy smokers and COPD patients. This resulted in a TGF-β-dependent increase in CXCL8 and IL-6 release, most notably in the cells from COPD patients. TGF-β stimulation increased SMAD3 expression, only in cells from COPD patients, with a concurrent increased miR-145 expression. Regulation of miR-145 was found to be negatively controlled by pathways involving the MAP kinases, MEK-1/2 and p38 MAPK. Subsequent, overexpression of miR-145 (using synthetic mimics) in ASM cells from patients with COPD suppressed IL-6 and CXCL8 release, to levels comparable to the nonsmoker controls. Therefore, this study suggests that miR-145 negatively regulates pro-inflammatory cytokine release from ASM cells in COPD by targeting SMAD3.
Keywords: COPD; inflammation; microRNA.
© 2016 The Authors. FEBS Letters published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Figures
Similar articles
-
Epigenetic Inhibitors Differentially Impact TGF-β1 Signaling Cascades in COPD Airway Smooth Muscle Cells.Cells. 2024 Dec 31;14(1):31. doi: 10.3390/cells14010031. Cells. 2024. PMID: 39791732 Free PMC article.
-
Oxidative stress-induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease.J Allergy Clin Immunol. 2015 Sep;136(3):769-80. doi: 10.1016/j.jaci.2015.01.046. Epub 2015 Mar 29. J Allergy Clin Immunol. 2015. PMID: 25828268 Free PMC article.
-
Decreased miR-29b expression is associated with airway inflammation in chronic obstructive pulmonary disease.Am J Physiol Lung Cell Mol Physiol. 2019 Apr 1;316(4):L621-L629. doi: 10.1152/ajplung.00436.2018. Epub 2019 Jan 17. Am J Physiol Lung Cell Mol Physiol. 2019. PMID: 30652495
-
The phosphoinositide 3'-kinase p110δ modulates contractile protein production and IL-6 release in human airway smooth muscle.J Cell Physiol. 2012 Aug;227(8):3044-52. doi: 10.1002/jcp.23046. J Cell Physiol. 2012. PMID: 22015454
-
The anti-proliferative and anti-inflammatory response of COPD airway smooth muscle cells to hydrogen sulfide.Respir Res. 2018 May 9;19(1):85. doi: 10.1186/s12931-018-0788-x. Respir Res. 2018. Retraction in: Respir Res. 2021 Aug 25;22(1):235. doi: 10.1186/s12931-021-01816-7. PMID: 29743070 Free PMC article. Retracted.
Cited by
-
Transforming Growth Factor-β1 Selectively Recruits microRNAs to the RNA-Induced Silencing Complex and Degrades CFTR mRNA under Permissive Conditions in Human Bronchial Epithelial Cells.Int J Mol Sci. 2019 Oct 5;20(19):4933. doi: 10.3390/ijms20194933. Int J Mol Sci. 2019. PMID: 31590401 Free PMC article.
-
Nanoparticle Delivery of Anti-inflammatory LNA Oligonucleotides Prevents Airway Inflammation in a HDM Model of Asthma.Mol Ther Nucleic Acids. 2020 Mar 6;19:1000-1014. doi: 10.1016/j.omtn.2019.12.033. Epub 2020 Jan 14. Mol Ther Nucleic Acids. 2020. PMID: 32044723 Free PMC article.
-
Inhaled RNA Therapeutics for Obstructive Airway Diseases: Recent Advances and Future Prospects.Pharmaceutics. 2021 Jan 28;13(2):177. doi: 10.3390/pharmaceutics13020177. Pharmaceutics. 2021. PMID: 33525500 Free PMC article. Review.
-
MicroRNAs as Potential Regulators of Immune Response Networks in Asthma and Chronic Obstructive Pulmonary Disease.Front Immunol. 2021 Jan 8;11:608666. doi: 10.3389/fimmu.2020.608666. eCollection 2020. Front Immunol. 2021. PMID: 33488613 Free PMC article. Review.
-
Chronic obstructive pulmonary disease: MicroRNAs and exosomes as new diagnostic and therapeutic biomarkers.J Res Med Sci. 2018 Mar 27;23:27. doi: 10.4103/jrms.JRMS_1054_17. eCollection 2018. J Res Med Sci. 2018. PMID: 29692824 Free PMC article. Review.
References
-
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, Menezes AM, Sullivan SD, Lee TA, Weiss KB et al (2007) International variation in the prevalence of COPD (the BOLD Study): a population‐based prevalence study. Lancet 370, 741–750. - PubMed
-
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M et al (2013) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 187, 347–365. - PubMed
-
- Barnes PJ (2014) Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin Chest Med 35, 71–86. - PubMed
-
- Jeffery PK (2004) Remodeling and inflammation of bronchi in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc 1, 176–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

