Mobile phone-based interventions for smoking cessation
- PMID: 27060875
- PMCID: PMC6485940
- DOI: 10.1002/14651858.CD006611.pub4
Mobile phone-based interventions for smoking cessation
Abstract
Background: Access to mobile phones continues to increase exponentially globally, outstripping access to fixed telephone lines, fixed computers and the Internet. Mobile phones are an appropriate and effective option for the delivery of smoking cessation support in some contexts. This review updates the evidence on the effectiveness of mobile phone-based smoking cessation interventions.
Objectives: To determine whether mobile phone-based smoking cessation interventions increase smoking cessation in people who smoke and want to quit.
Search methods: For the most recent update, we searched the Cochrane Tobacco Addiction Group Specialised Register in April 2015. We also searched the UK Clinical Research Network Portfolio for current projects in the UK, and the ClinicalTrials.gov register for ongoing or recently completed studies. We searched through the reference lists of identified studies and attempted to contact the authors of ongoing studies. We applied no restrictions on language or publication date.
Selection criteria: We included randomised or quasi-randomised trials. Participants were smokers of any age who wanted to quit. Studies were those examining any type of mobile phone-based intervention for smoking cessation. This included any intervention aimed at mobile phone users, based around delivery via mobile phone, and using any functions or applications that can be used or sent via a mobile phone.
Data collection and analysis: Review authors extracted information on risk of bias and methodological details using a standardised form. We considered participants who dropped out of the trials or were lost to follow-up to be smoking. We calculated risk ratios (RR) and 95% confidence intervals (CI) for each included study. Meta-analysis of the included studies used the Mantel-Haenszel fixed-effect method. Where meta-analysis was not possible, we presented a narrative summary and descriptive statistics.
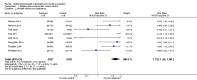
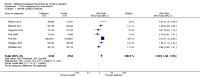
Main results: This updated search identified 12 studies with six-month smoking cessation outcomes, including seven studies completed since the previous review. The interventions were predominantly text messaging-based, although several paired text messaging with in-person visits or initial assessments. Two studies gave pre-paid mobile phones to low-income human immunodeficiency virus (HIV)-positive populations - one solely for phone counselling, the other also included text messaging. One study used text messages to link to video messages. Control programmes varied widely. Studies were pooled according to outcomes - some providing measures of continuous abstinence or repeated measures of point prevalence; others only providing 7-day point prevalence abstinence. All 12 studies pooled using their most rigorous 26-week measures of abstinence provided an RR of 1.67 (95% CI 1.46 to 1.90; I(2) = 59%). Six studies verified quitting biochemically at six months (RR 1.83; 95% CI 1.54 to 2.19).
Authors' conclusions: The current evidence supports a beneficial impact of mobile phone-based smoking cessation interventions on six-month cessation outcomes. While all studies were good quality, the fact that those studies with biochemical verification of quitting status demonstrated an even higher chance of quitting further supports the positive findings. However, it should be noted that most included studies were of text message interventions in high-income countries with good tobacco control policies. Therefore, caution should be taken in generalising these results outside of this type of intervention and context.
Conflict of interest statement
RW was co‐author of one paper on one of the included studies (Bramley 2005). She was a co‐investigator on two included studies (Free 2009; Free 2011), and principle investigator of a further included study (Whittaker 2011).
CB and HM were co‐authors of Whittaker 2011.
RB was co‐author of one of the trials (Borland 2013), and he led the development of the intervention.
AR was a lead author (Rodgers 2005), and a co‐author (Free 2009; Free 2011; Whittaker 2011), on included studies.
HM received honoraria from Johnson & Johnson and Pfizer for speaking at educational events and attending advisory group meetings. He has also received investigator initiated research funding from Pfizer.
RW's institution has received grant money to cover the costs of providing the text messaging intervention for the study described in Free 2011, and there is a grant pending to develop this intervention further for a different audience. The institution has also licensed the STOMP intervention described in Rodgers 2005 to HSAGlobal.
All other authors had no other known conflicts of interest.
Figures







Update of
-
Mobile phone-based interventions for smoking cessation.Cochrane Database Syst Rev. 2012 Nov 14;11:CD006611. doi: 10.1002/14651858.CD006611.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2016 Apr 10;4:CD006611. doi: 10.1002/14651858.CD006611.pub4. PMID: 23152238 Updated.
References
References to studies included in this review
-
- Abroms L, Ahuja M, Kodl Y, Thaweethai L, Sims J, Winickoff J, et al. Text2Quit: results from a pilot test of a personalised, interactive mobile health smoking cessation program. Journal of Health Communication 2012;17(Suppl 1):44‐53. - PMC - PubMed
- Abroms LC, Boal AL, Simmens SJ, Mendel JA, Windsor RA. A randomized trial of Text2Quit: a text messaging program for smoking cessation. American Journal of Preventive Medicine 2014;47(3):242‐50. [CENTRAL: 1002545; CRS: 9400129000003040; EMBASE: 2014941087] - PMC - PubMed
-
- Balmford J, Borland R, Benda P, Howard S. Factors associated with use of automated smoking cessation interventions: findings from the eQuit study. Health Education Research 2013;28(2):288‐99. [CENTRAL: 921007; CRS: 9400107000001543; PUBMED: 23107931] - PubMed
- Borland R, Balmford J, Benda P. Population‐level effects of automated smoking cessation help programs: a randomized controlled trial. Addiction (Abingdon, England) 2013;108(3):618‐28. [CENTRAL: 921001; CRS: 9400107000000767; PUBMED: 22994457] - PubMed
-
- Ferguson SG, Walters JAE. The effect of mobile phone text messages on short and long term quitting in motivated smokers: a randomised controlled trial. Proceedings of the Society for Research on Nicotine and Tobacco 21st Annual Meeting; 2015 Feb 25‐28 Philadelphia. 2015:252 (POS3‐80). [CRS: 9400131000001072]
- Schuz N, Walters JAE, Frandsen M, Bower J, Ferguson SG. Compliance with an EMA monitoring protocol and its relationship with participant and smoking characteristics. Nicotine & Tobacco Research 2014;16(Suppl 2):S88‐92. [CRS: 9400129000001511; EMBASE: 2014262309] - PubMed
-
- Free C, Whittaker R, Knight R, Abramsky T, Rodgers A, Roberts IG. Txt2stop: a pilot randomised controlled trial of mobile phone‐based smoking cessation support. Tobacco Control 2009;18:88‐91. - PubMed
References to studies excluded from this review
-
- Applegate BW, Raymond C, Collado‐Rodriguez A, Riley WT, Schneider NG. Improving adherence to nicotine gum by sms text messaging: a pilot study (RPOS3‐57). Proceedings of the Society for Research on Nicotine and Tobacco, 13th Annual Meeting; 2007 Feb 21‐24; Austin, Texas. 2007:14.
-
- Bennett DA, Emberson JR. Text messaging in smoking cessation: the Txt2stop trial [Comment]. Lancet 2011;378(9785):6‐7. - PubMed
-
- Blasco A, Carmona M, Fernandez‐Lozano I, Salvador CH, Pascual M, Sagredo PG, et al. Evaluation of a telemedicine service for the secondary prevention of coronary artery disease. Journal of Cardiopulmonary Rehabilitation and Prevention 2012;32(1):25‐31. [CENTRAL: 814645; CRS: 9400123000012404; 6831; PUBMED: 22113368] - PubMed
-
- Brendryen H, Kraft P. Happy Ending: a randomized controlled trial of a digital multi‐media smoking cessation intervention. Addiction 2008;103:478‐84. [doi:10.1111/j.1360‐0443.2007.02119.x] - PubMed
References to ongoing studies
-
- NCT01103427. Mobile text messaging as an adjunct function to an Internet‐based smoking cessation intervention implemented in the general population and in a health care setting, 2010. clinicaltrials.gov/show/NCT01103427 (accessed 5 April 2016). [CRS: 9400131000001046]
-
- NCT01723163. Abstinence reinforcement therapy (ART) for rural veteran smokers, 2013. clinicaltrials.gov/show/NCT01723163 (accessed 5 April 2016). [CRS: 9400131000001061]
-
- NCT01746069. Evaluation of a reinforcement program using text messages through mobile phone in smoking cessation programs in primary care, 2013. clinicaltrials.gov/show/NCT01746069 (accessed 5 April 2016). [CRS: 9400131000001033]
Additional references
-
- Bramley D, Riddell T, Whittaker R, Corbett T, Lin R‐B, Wills M. Smoking cessation using mobile phone text messaging is as effective in Maori as non‐Maori. New Zealand Medical Journal 2005;118(1216):1494‐504. - PubMed
-
- Ericsson. Ericsson mobility report on the pulse of the networked society, 2015. www.ericsson.com/ericsson‐mobility‐report (accessed 5 April 2016).
-
- Fiore M, Jaén C, Baker T. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services, Public Health Service, 2008.
References to other published versions of this review
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

