Establishing the first institutional animal care and use committee in Egypt
- PMID: 27060909
- PMCID: PMC4826520
- DOI: 10.1186/s13010-016-0035-3
Establishing the first institutional animal care and use committee in Egypt
Abstract
Background: Although animal research ethics committees (AREC) are well established in Western countries, this field is weakly developed and its concept is poorly understood in the Middle East and North Africa region.
Objective: Our main objective was to introduce the concept and requirements of ethical approaches in dealing with experimental animal in research and teaching in Egypt.
Methods: Due to its very recent inception, Cairo University, Faculty of Science IACUC decided to operate in accordance with Guide for the Care and Use of Laboratory Animals 8th Edition 2011 (the Guide) since Egypt has not yet compiled its own guide.
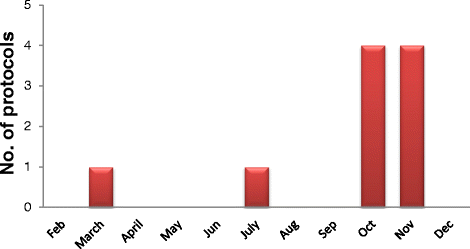
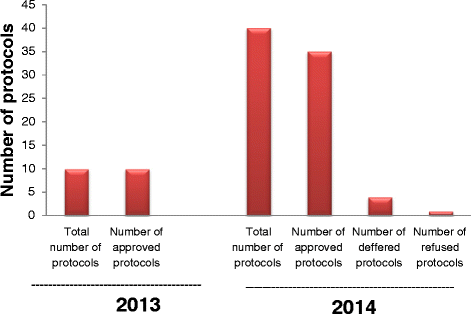
Results: Fifty protocols were reviewed in 2013-2014. Only ten protocols were reviewed in 2013, but in 2014, forty protocols were reviewed. In 2013 all protocols were approved and in 2014, number of approvals were 35, the number of deferrals were 4, and one refused protocol. Master's theses (MSc) research protocols constituted the majority of the total reviewed protocols. This is attributed to the decision of the Board of the Faculty of Science, Cairo University in September, 2013 that the approval of the IACUC is mandatory before conducting any research involving animals or theses registration.
Conclusion: The first IACUC was established in the Cairo University, Faculty of Science, since 2012. The challenges encountered by the committee were diverse, such as the absence of laws that control the use of animal models in scientific research, lack of guidelines (protocols for experimental animals in research) and, mandatory ethical approval for any experimental animal research.
Figures
Similar articles
-
The status and issues of the Institutional Animal Care and Use Committee of Seoul National University: from its establishment to the present day.Exp Anim. 2021 Nov 10;70(4):532-540. doi: 10.1538/expanim.21-0066. Epub 2021 Jun 30. Exp Anim. 2021. PMID: 34193732 Free PMC article.
-
Ethics: views from IACUC members.Altern Lab Anim. 2009 Jul;37(3):291-6. doi: 10.1177/026119290903700311. Altern Lab Anim. 2009. PMID: 19678730
-
Protocol audits for post-approval monitoring of animal use protocols.Lab Anim (NY). 2005 Nov;34(10):23-7. doi: 10.1038/laban1105-23. Lab Anim (NY). 2005. PMID: 16261150
-
What investigators need to know about the use of animals.ILAR J. 2014;54(3):324-8. doi: 10.1093/ilar/ilt046. ILAR J. 2014. PMID: 24615446 Review.
-
Animal research ethics in Africa: an overview.Acta Trop. 2009 Nov;112 Suppl 1:S48-52. doi: 10.1016/j.actatropica.2009.07.021. Epub 2009 Jul 30. Acta Trop. 2009. PMID: 19646942 Review.
Cited by
-
Montelukast potentiates the antiinflammatory effect of NSAIDs in the rat paw formalin model and simultaneously minimizes the risk of gastric damage.Inflamm Res. 2021 Sep;70(9):981-992. doi: 10.1007/s00011-021-01492-9. Epub 2021 Aug 11. Inflamm Res. 2021. PMID: 34382102
-
Enhanced mitochondrial biogenesis is associated with the ameliorative action of creatine supplementation in rat soleus and cardiac muscles.Exp Ther Med. 2020 Jan;19(1):384-392. doi: 10.3892/etm.2019.8173. Epub 2019 Nov 7. Exp Ther Med. 2020. PMID: 31853315 Free PMC article.
-
Biophysical, histological, and bioaccumulation properties of Tilapia muscle affected by water pollution with heavy elements and microbes at the El-Rahawy drain in Egypt.Heliyon. 2023 Mar 15;9(3):e14489. doi: 10.1016/j.heliyon.2023.e14489. eCollection 2023 Mar. Heliyon. 2023. PMID: 36967882 Free PMC article.
-
tert-Butylhydroquinone Treatment Alleviates Contrast-Induced Nephropathy in Rats by Activating the Nrf2/Sirt3/SOD2 Signaling Pathway.Oxid Med Cell Longev. 2019 Dec 18;2019:4657651. doi: 10.1155/2019/4657651. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31929854 Free PMC article.
-
Gastroprotective Effects of Betahistine Against an Indomethacin-Induced Gastric Mucosal Ulcer in Rats: The Role of CINC-2α Gene.Med J Islam Repub Iran. 2024 Sep 2;38:100. doi: 10.47176/mjiri.38.100. eCollection 2024. Med J Islam Repub Iran. 2024. PMID: 39678770 Free PMC article.
References
-
- Abdel-Aal W, Abdel Ghaffar E, El Shabrawy O. Review of the medical research ethics committee (MREC), national research center of Egypt. Curr Med Res Opin. 2003;2013:1–7. - PubMed
-
- Bayne K. Animal care and use programs: Global harmonization through alternatives. Proc. 6th World Congress on Alternatives & Animal Use in the Life Sciences. 2007; 749–752
-
- Anon. Annexes to the Egyptian Law of Agriculture No. 53/1966. Egyptian off. Gazette (El Wakkea El Masrya) 1967.
-
- The 2014 Egyptian constitution article 45. http://www.sis.gov.eg/Newvr/Dustor-en001.pdf.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources