Combining an epithelial repair factor and anti-fibrotic with a corticosteroid offers optimal treatment for allergic airways disease
- PMID: 27060978
- PMCID: PMC4882488
- DOI: 10.1111/bph.13494
Combining an epithelial repair factor and anti-fibrotic with a corticosteroid offers optimal treatment for allergic airways disease
Abstract
Background and purpose: We evaluated the extent to which individual versus combination treatments that specifically target airway epithelial damage [trefoil factor-2 (TFF2)], airway fibrosis [serelaxin (RLX)] or airway inflammation [dexamethasone (DEX)] reversed the pathogenesis of chronic allergic airways disease (AAD).
Experimental approach: Following induction of ovalbumin (OVA)-induced chronic AAD in 6–8 week female Balb/c mice, animals were i.p. administered naphthalene (NA) on day 64 to induce epithelial damage, then received daily intranasal administration of RLX (0.8 mg·mL(−1)), TFF2 (0.5 mg·mL(−1)), DEX (0.5 mg·mL(−1)), RLX + TFF2 or RLX + TFF2 + DEX from days 67–74. On day 75, lung function was assessed by invasive plethysmography, before lung tissue was isolated for analyses of various measures. The control group was treated with saline + corn oil (vehicle for NA).
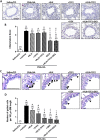
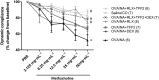
Key results: OVA + NA-injured mice demonstrated significantly increased airway inflammation, airway remodelling (AWR) (epithelial damage/thickness; subepithelial myofibroblast differentiation, extracellular matrix accumulation and fibronectin deposition; total lung collagen concentration), and significantly reduced airway dynamic compliance (cDyn). RLX + TFF2 markedly reversed several measures of OVA + NA-induced AWR and normalized the reduction in cDyn. The combined effects of RLX + TFF2 + DEX significantly reversed peribronchial inflammation score, airway epithelial damage, subepithelial extracellular matrix accumulation/fibronectin deposition and total lung collagen concentration (by 50–90%) and also normalized the reduction of cDyn.
Conclusions and implications: Combining an epithelial repair factor and anti-fibrotic provides an effective means of treating the AWR and dysfunction associated with AAD/asthma and may act as an effective adjunct therapy to anti-inflammatory corticosteroids
Background and Purpose: We evaluated the extent to which individual versus combination treatments that specifically target airway epithelial damage [trefoil factor‐2 (TFF2)], airway fibrosis [serelaxin (RLX)] or airway inflammation [dexamethasone (DEX)] reversed the pathogenesis of chronic allergic airways disease (AAD).
Experimental Approach: Following induction of ovalbumin (OVA)‐induced chronic AAD in 6–8 week female Balb/c mice, animals were i.p. administered naphthalene (NA) on day 64 to induce epithelial damage, then received daily intranasal administration of RLX (0.8 mg·mL−1), TFF2 (0.5 mg·mL−1), DEX (0.5 mg·mL−1), RLX + TFF2 or RLX + TFF2 + DEX from days 67–74. On day 75, lung function was assessed by invasive plethysmography, before lung tissue was isolated for analyses of various measures. The control group was treated with saline + corn oil (vehicle for NA).
Key Results: OVA + NA‐injured mice demonstrated significantly increased airway inflammation, airway remodelling (AWR) (epithelial damage/thickness; subepithelial myofibroblast differentiation, extracellular matrix accumulation and fibronectin deposition; total lung collagen concentration), and significantly reduced airway dynamic compliance (cDyn). RLX + TFF2 markedly reversed several measures of OVA + NA‐induced AWR and normalized the reduction in cDyn. The combined effects of RLX + TFF2 + DEX significantly reversed peribronchial inflammation score, airway epithelial damage, subepithelial extracellular matrix accumulation/fibronectin deposition and total lung collagen concentration (by 50–90%) and also normalized the reduction of cDyn.
Conclusions and Implications: Combining an epithelial repair factor and anti‐fibrotic provides an effective means of treating the AWR and dysfunction associated with AAD/asthma and may act as an effective adjunct therapy to anti‐inflammatory corticosteroids.
Figures




Similar articles
-
Serelaxin improves the therapeutic efficacy of RXFP1-expressing human amnion epithelial cells in experimental allergic airway disease.Clin Sci (Lond). 2016 Dec 1;130(23):2151-2165. doi: 10.1042/CS20160328. Epub 2016 Sep 19. Clin Sci (Lond). 2016. PMID: 27647937
-
Characterization of a novel model incorporating airway epithelial damage and related fibrosis to the pathogenesis of asthma.Lab Invest. 2014 Dec;94(12):1326-39. doi: 10.1038/labinvest.2014.119. Epub 2014 Sep 29. Lab Invest. 2014. PMID: 25264707
-
Trefoil factor-2 reverses airway remodeling changes in allergic airways disease.Am J Respir Cell Mol Biol. 2013 Jan;48(1):135-44. doi: 10.1165/rcmb.2011-0320OC. Epub 2012 May 31. Am J Respir Cell Mol Biol. 2013. PMID: 22652198
-
iPSC- and mesenchymoangioblast-derived mesenchymal stem cells provide greater protection against experimental chronic allergic airways disease compared with a clinically used corticosteroid.FASEB J. 2019 May;33(5):6402-6411. doi: 10.1096/fj.201802307R. Epub 2019 Feb 15. FASEB J. 2019. PMID: 30768365
-
Intranasally administered serelaxin abrogates airway remodelling and attenuates airway hyperresponsiveness in allergic airways disease.Clin Exp Allergy. 2014 Nov;44(11):1399-408. doi: 10.1111/cea.12391. Clin Exp Allergy. 2014. PMID: 25113628
Cited by
-
Serelaxin enhances the therapeutic effects of human amnion epithelial cell-derived exosomes in experimental models of lung disease.Br J Pharmacol. 2019 Jul;176(13):2195-2208. doi: 10.1111/bph.14666. Epub 2019 May 7. Br J Pharmacol. 2019. PMID: 30883698 Free PMC article.
-
The renoprotective efficacy and safety of genetically-engineered human bone marrow-derived mesenchymal stromal cells expressing anti-fibrotic cargo.Stem Cell Res Ther. 2024 Oct 23;15(1):375. doi: 10.1186/s13287-024-03992-x. Stem Cell Res Ther. 2024. PMID: 39443975 Free PMC article.
-
The novel AT2 receptor ligand, β-Pro7 Ang III, induces equivalent anti-fibrotic effects to Compound 21 but broader anti-fibrotic effects than pirfenidone in mice with bleomycin-induced pulmonary fibrosis.Clin Sci (Lond). 2025 Jul 31;139(14):809-824. doi: 10.1042/CS20245138. Clin Sci (Lond). 2025. PMID: 40590236 Free PMC article.
References
-
- Baccari MC, Bani D, Bigazzi M, Calamai F (2004). Influence of relaxin on the neurally induced relaxant responses of the mouse gastric fundus. Biol Reprod 71: 1325–1329. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

