Anti-stromal treatment together with chemotherapy targets multiple signalling pathways in pancreatic adenocarcinoma
- PMID: 27061193
- PMCID: PMC5025731
- DOI: 10.1002/path.4727
Anti-stromal treatment together with chemotherapy targets multiple signalling pathways in pancreatic adenocarcinoma
Abstract
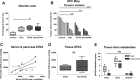
Stromal targeting for pancreatic ductal adenocarcinoma (PDAC) is rapidly becoming an attractive option, due to the lack of efficacy of standard chemotherapy and increased knowledge about PDAC stroma. We postulated that the addition of stromal therapy may enhance the anti-tumour efficacy of chemotherapy. Gemcitabine and all-trans retinoic acid (ATRA) were combined in a clinically applicable regimen, to target cancer cells and pancreatic stellate cells (PSCs) respectively, in 3D organotypic culture models and genetically engineered mice (LSL-Kras(G12D) (/+) ;LSL-Trp53(R172H) (/+) ;Pdx-1-Cre: KPC mice) representing the spectrum of PDAC. In two distinct sets of organotypic models as well as KPC mice, we demonstrate a reduction in cancer cell proliferation and invasion together with enhanced cancer cell apoptosis when ATRA is combined with gemcitabine, compared to vehicle or either agent alone. Simultaneously, PSC activity (as measured by deposition of extracellular matrix proteins such as collagen and fibronectin) and PSC invasive ability were both diminished in response to combination therapy. These effects were mediated through a range of signalling cascades (Wnt, hedgehog, retinoid, and FGF) in cancer as well as stellate cells, affecting epithelial cellular functions such as epithelial-mesenchymal transition, cellular polarity, and lumen formation. At the tissue level, this resulted in enhanced tumour necrosis, increased vascularity, and diminished hypoxia. Consequently, there was an overall reduction in tumour size. The enhanced effect of stromal co-targeting (ATRA) alongside chemotherapy (gemcitabine) appears to be mediated by dampening multiple signalling cascades in the tumour-stroma cross-talk, rather than ablating stroma or targeting a single pathway. © 2016 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Keywords: all-trans-retinoic acid; collagen; fibronectin; gemcitabine; pancreatic stellate cells; quiescence.
© 2016 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Figures





References
-
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364 : 1817–1825. - PubMed
-
- National Institute for Health and Care Excellence . The use of gemcitabine for the treatment of pancreatic cancer (TA25). 2001. [Accessed 1 February 2016]. Available from: https://www.nice.org.uk/guidance/ta25
-
- Neesse A, Algul H, Tuveson DA, et al. Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut 2015; 64 : 1476–1484. - PubMed
-
- Neesse A, Michl P, Frese KK, et al. Stromal biology and therapy in pancreatic cancer. Gut 2011; 60: 861–868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

