Photoelectrochemical H2 Evolution with a Hydrogenase Immobilized on a TiO2 -Protected Silicon Electrode
- PMID: 27061334
- PMCID: PMC4981910
- DOI: 10.1002/anie.201511822
Photoelectrochemical H2 Evolution with a Hydrogenase Immobilized on a TiO2 -Protected Silicon Electrode
Abstract
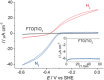
The combination of enzymes with semiconductors enables the photoelectrochemical characterization of electron-transfer processes at highly active and well-defined catalytic sites on a light-harvesting electrode surface. Herein, we report the integration of a hydrogenase on a TiO2 -coated p-Si photocathode for the photo-reduction of protons to H2 . The immobilized hydrogenase exhibits activity on Si attributable to a bifunctional TiO2 layer, which protects the Si electrode from oxidation and acts as a biocompatible support layer for the productive adsorption of the enzyme. The p-Si|TiO2 |hydrogenase photocathode displays visible-light driven production of H2 at an energy-storing, positive electrochemical potential and an essentially quantitative faradaic efficiency. We have thus established a widely applicable platform to wire redox enzymes in an active configuration on a p-type semiconductor photocathode through the engineering of the enzyme-materials interface.
Keywords: TiO2; hydrogen evolution; hydrogenase; photoelectrochemistry; semiconductors.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


References
-
- None
-
- Lubitz W., Ogata H., Rüdiger O., Reijerse E., Chem. Rev. 2014, 114, 4081–4148; - PubMed
-
- Vincent K. A., Parkin A., Armstrong F. A., Chem. Rev. 2007, 107, 4366–4413; - PubMed
-
- Jones A. K., Sillery E., Albracht S. P. J., Armstrong F. A., Chem. Commun. 2002, 866–867; - PubMed
-
- Hamdan A. A., Dementin S., Liebgott P.-P., Gutierrez-Sanz O., Richaud P., De Lacey A. L., Rousset M., Bertrand P., Cournac L., Léger C., J. Am. Chem. Soc. 2012, 134, 8368–8371; - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

