Nonrandomized comparison of neurofibromatosis type 1 and non-neurofibromatosis type 1 children who received carboplatin and vincristine for progressive low-grade glioma: A report from the Children's Oncology Group
- PMID: 27061921
- PMCID: PMC4892942
- DOI: 10.1002/cncr.29987
Nonrandomized comparison of neurofibromatosis type 1 and non-neurofibromatosis type 1 children who received carboplatin and vincristine for progressive low-grade glioma: A report from the Children's Oncology Group
Abstract
Background: To evaluate tumor responses, event-free survival (EFS), overall survival (OS), and toxicity of chemotherapy, children with neurofibromatosis type 1 (NF1) and progressive low-grade glioma were enrolled into the Children's Oncology Group (COG) A9952 protocol and treated with carboplatin and vincristine (CV).
Methods: Non-NF1 patients were randomized to CV or thioguanine, procarbazine, 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea, and vincristine in COG A9952. NF1 patients were assigned to CV only. NF1 patients and non-NF1 patients who were treated with CV were compared with respect to baseline characteristics, toxicity, tumor responses, EFS, and OS.
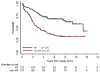
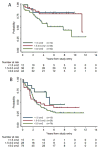
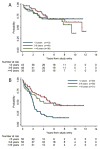
Results: A total of 127 eligible patients with NF1 were nonrandomly assigned to CV: 42 NF1 patients (33%) had events, and 6 (4.7%) died. The 5-year EFS rate was 69% ± 4% for the CV-NF1 group and 39% ± 4% for the CV-non-NF1 group (P < .001). In a univariate analysis, NF1 children had a significantly higher tumor response rate and superior EFS and OS in comparison with CV-treated children without NF1. NF1 patients and non-NF1 patients differed significantly in amount of residual tumor, extent of resection, tumor location, and pathology. According to a multivariate analysis, NF1 was independently associated with better EFS (P < .001) but not with OS. NF1 patients also had a decreased risk of grade 3 or 4 toxicities in comparison with non-NF1 patients. Three second malignant neoplasms occurred in NF1 patients receiving CV (CV-NF1 group) at a median of 7.8 years (range, 7.3-9.4 years) after enrollment, but there were none in the non-NF1 group.
Conclusions: Children with NF1 tolerated CV well and had tumor response rates and EFS that were superior to those for children without NF1. Cancer 2016;122:1928-36. © 2016 American Cancer Society.
Keywords: carboplatin; childhood low-grade glioma; neurofibromatosis 1; pilocytic astrocytoma; vincristine.
© 2016 American Cancer Society.
Conflict of interest statement
There are no financial disclosures or conflicts of interest from any authors, except the following:
Roger Packer, MD has had travel expenses supported by AstraZeneca
Figures

References
-
- Listernick R, Charrow J, Gutmann DH. Intracranial gliomas in neurofibromatosis type 1. Am J Med Genet. 1999;89:38–44. - PubMed
-
- Listernick R, Charrow J, Greenwald MJ, Esterly NB. Optic gliomas in children with neurofibromatosis type 1. J Pediatr. 1989;114:788–792. - PubMed
-
- Janss AJ, Grundy R, Cnaan A, et al. Optic pathway and hypothalamic/chiasmatic gliomas in children younger than age 5 years with a 6-year follow-up. Cancer. 1995;75:1051–1059. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

