Regression Rates Following the Treatment of Aggressive Posterior Retinopathy of Prematurity with Bevacizumab Versus Laser: 8-Year Retrospective Analysis
- PMID: 27062023
- PMCID: PMC4918525
- DOI: 10.12659/msm.897095
Regression Rates Following the Treatment of Aggressive Posterior Retinopathy of Prematurity with Bevacizumab Versus Laser: 8-Year Retrospective Analysis
Abstract
BACKGROUND Retinopathy is a serious complication related to prematurity and a leading cause of childhood blindness. The aggressive posterior form of retinopathy of prematurity (APROP) has a worse anatomical and functional outcome following laser therapy, as compared with the classic form of the disease. The main outcome measures are the APROP regression rate, structural outcomes, and complications associated with intravitreal bevacizumab (IVB) versus laser photocoagulation in APROP. MATERIAL AND METHODS This is a retrospective case series that includes infants with APROP who received either IVB or laser photocoagulation and had a follow-up of at least 60 weeks (for the laser photocoagulation group) and 80 weeks (for the IVB group). In the first group, laser photocoagulation of the retina was carried out and in the second group, 1 bevacizumab injection was administered intravitreally. The following parameters were analyzed in each group: sex, gestational age, birth weight, postnatal age and postmenstrual age at treatment, APROP regression, sequelae, and complications. Statistical analysis was performed using Microsoft Excel and IBM SPSS (version 23.0). RESULTS The laser photocoagulation group consisted of 6 premature infants (12 eyes) and the IVB group consisted of 17 premature infants (34 eyes). Within the laser photocoagulation group, the evolution was favorable in 9 eyes (75%) and unfavorable in 3 eyes (25%). Within the IVB group, APROP regressed in 29 eyes (85.29%) and failed to regress in 5 eyes (14.71%). These differences are statistically significant, as proved by the McNemar test (P<0.001). CONCLUSIONS The IVB group had a statistically significant better outcome compared with the laser photocoagulation group, in APROP in our series.
Figures
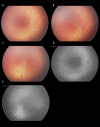
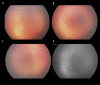
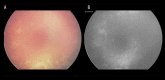
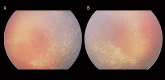
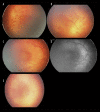
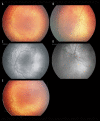
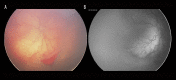
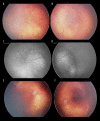
Similar articles
-
Comparison of Outcomes Between Combined Sparing Laser Photocoagulation and Intravitreal Bevacizumab Treatment Versus Conventional Laser Photocoagulation in Aggressive Posterior Retinopathy of Prematurity.J Pediatr Ophthalmol Strabismus. 2021 Sep-Oct;58(5):292-297. doi: 10.3928/01913913-20210316-01. Epub 2021 Sep 1. J Pediatr Ophthalmol Strabismus. 2021. PMID: 34180288
-
Outcomes after Intravitreal Bevacizumab versus Laser Photocoagulation for Retinopathy of Prematurity: A 5-Year Retrospective Analysis.Ophthalmology. 2015 May;122(5):1008-15. doi: 10.1016/j.ophtha.2014.12.017. Epub 2015 Feb 14. Ophthalmology. 2015. PMID: 25687024 Free PMC article.
-
Evaluation of 2-year outcomes following intravitreal bevacizumab (IVB) for aggressive posterior retinopathy of prematurity.Arq Bras Oftalmol. 2015 Sep-Oct;78(5):300-4. doi: 10.5935/0004-2749.20150079. Arq Bras Oftalmol. 2015. PMID: 26466229
-
Development of refractive error in children treated for retinopathy of prematurity with anti-vascular endothelial growth factor (anti-VEGF) agents: A meta-analysis and systematic review.PLoS One. 2019 Dec 2;14(12):e0225643. doi: 10.1371/journal.pone.0225643. eCollection 2019. PLoS One. 2019. PMID: 31790445 Free PMC article.
-
Aggressive posterior retinopathy of prematurity: a review on current understanding.Eye (Lond). 2021 Apr;35(4):1140-1158. doi: 10.1038/s41433-021-01392-6. Epub 2021 Jan 29. Eye (Lond). 2021. PMID: 33514899 Free PMC article. Review.
Cited by
-
The efficacy and ocular safety following aflibercept, conbercept, ranibizumab, bevacizumab, and laser for retinopathy of prematurity: a systematic review and meta-analysis.Ital J Pediatr. 2023 Oct 9;49(1):136. doi: 10.1186/s13052-023-01543-3. Ital J Pediatr. 2023. PMID: 37814332 Free PMC article.
-
[Review of clinical trials in retinopathy of prematurity : Current state and future perspectives].Ophthalmologe. 2018 Jun;115(6):456-463. doi: 10.1007/s00347-018-0720-2. Ophthalmologe. 2018. PMID: 29789899 Review. German.
-
Anti-vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity.Cochrane Database Syst Rev. 2018 Jan 8;1(1):CD009734. doi: 10.1002/14651858.CD009734.pub3. Cochrane Database Syst Rev. 2018. PMID: 29308602 Free PMC article.
-
[Treated cases of retinopathy of prematurity in Germany : 5-year data from the Retina.net ROP registry].Ophthalmologe. 2018 Jun;115(6):476-488. doi: 10.1007/s00347-018-0701-5. Ophthalmologe. 2018. PMID: 29637302 German.
-
Bevacizumab or laser for aggressive posterior retinopathy of prematurity.Taiwan J Ophthalmol. 2018 Oct-Dec;8(4):243-248. doi: 10.4103/tjo.tjo_69_18. Taiwan J Ophthalmol. 2018. PMID: 30637196 Free PMC article.
References
-
- Stenkuller PG, Du L, Gilbert C, et al. Childhood blindness. J AAPOS. 1999;3:26–32. - PubMed
-
- Gilbert C, Fielder A, Gordillo L, et al. International NO-ROP Group. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programs. Pediatrics. 2005;115(5):e518–25. - PubMed
-
- Clark D, Mandal K. Treatment of retinopathy of prematurity. Early Hum Dev. 2008;84:95–99. - PubMed
-
- Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indication for the treatment of retinopathy of prematurity: Results of early treatment for ROP randomized trial. Arch Ophthalmol. 2003;121:1684–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

