Comparison of Drospirenone- with Cyproterone Acetate-Containing Oral Contraceptives, Combined with Metformin and Lifestyle Modifications in Women with Polycystic Ovary Syndrome and Metabolic Disorders: A Prospective Randomized Control Trial
- PMID: 27064030
- PMCID: PMC4831520
- DOI: 10.4103/0366-6999.179783
Comparison of Drospirenone- with Cyproterone Acetate-Containing Oral Contraceptives, Combined with Metformin and Lifestyle Modifications in Women with Polycystic Ovary Syndrome and Metabolic Disorders: A Prospective Randomized Control Trial
Abstract
Background: While combined oral contraceptives (COCs) are commonly used to treat polycystic ovary syndrome (PCOS), comparative data regarding metabolic effects of different progestogens on this patient population are missing. This study aimed to compare the different effects of drospirenone (DRP)-containing COCs with cyproterone acetate (CPA)-containing COCs, combined with metformin and lifestyle modifications in women with PCOS and metabolic disorders.
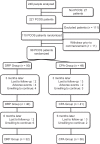
Methods: Ninety-nine women with PCOS and a metabolic disorder between January 2011 and January 2013 were enrolled into this prospective randomized clinical trial. Participants were randomized into two groups such as DRP-containing COCs, and CPA-containing COCs. Participants took COCs cyclically for 6 months, combined with metformin administration (1.5 g/d) and lifestyle modifications (diet and exercise). Clinical measures and biochemical and hormone profiles were compared. Comparisons for continuous variables were evaluated with paired and unpaired Student's t-tests. The Wilcoxon signed rank test was used when the data were not normally distributed. Analysis of covariance was used to control for age, body mass index (BMI), and baseline data of each analyzed parameter when compared between the two groups.
Results: A total of 68 patients have completed the study. The combination regimen of COCs, metformin, and lifestyle modifications in these patients resulted in a significant decrease in BMI, acne, and hirsutism scores when compared to baseline levels in both groups (P < 0.05). Blood pressure (BP) was significantly different in the CPA group when compared to baseline (75.14 ± 6.77 mmHg vs. 80.70 ± 5.60 mmHg, P < 0.01), and after 6 months of treatment, only the change in systolic BP was significantly different between the two groups (4.00 [-6.00, 13.00] mmHg vs. -3.50 [-13.00, 9.00] mmHg, P = 0.009). Fasting glucose, fasting insulin, and homeostasis model assessment-insulin resistance decreased significantly in the DRP group (5.40 ± 0.41 mmol/L vs. 5.21 ± 0.32 mmol/L, P = 0.041; 13.90 [10.50, 18.40] μU/ml vs. 10.75 [8.60, 13.50] μU/ml, P = 0.020; 3.74 [2.85, 4.23] vs. 2.55 [1.92, 3.40], P = 0.008) but did not differ between the two groups. While individual lipid profiles increased in both groups, no statistically significant difference was observed.
Conclusions: DRP-containing COCs combined with metformin and lifestyle modifications could better control BP and correct carbohydrate metabolism in women with PCOS and metabolic disorders compared with CPA-containing COCs.
Trial registration: Chinese Clinical Trial Registry, ChiCTR-TRC-11001143; http://www.chictr.org.cn/showproj.aspx?proj=8395.
Figures
References
-
- Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod Update. 2010;16:347–63. doi:10.1093/humupd/dmq001. - PubMed
-
- Chun-Sen H, Chien-Hua W, Wan-Chun C, Ching-Tzu L, Chun-Jen C, Ming IH. Obesity and insulin resistance in women with polycystic ovary syndrome. Gynecol Endocrinol. 2011;27:300–6. doi:10.3109/09513590.2010.488776. - PubMed
-
- Liou TH, Yang JH, Hsieh CH, Lee CY, Hsu CS, Hsu MI. Clinical and biochemical presentations of polycystic ovary syndrome among obese and nonobese women. Fertil Steril. 2009;92:1960–5. doi:10.1016/j.fertnstert.2008.09.003. - PubMed
-
- Pasquali R. Obesity and androgens: Facts and perspectives. Fertil Steril. 2006;85:1319–40. doi:10.1016/j.fertnstert.2005.10.054. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical