In Vivo Amyloid-β Imaging in the APPPS1-21 Transgenic Mouse Model with a (89)Zr-Labeled Monoclonal Antibody
- PMID: 27064204
- PMCID: PMC4815004
- DOI: 10.3389/fnagi.2016.00067
In Vivo Amyloid-β Imaging in the APPPS1-21 Transgenic Mouse Model with a (89)Zr-Labeled Monoclonal Antibody
Abstract
Introduction: The accumulation of amyloid-β is a pathological hallmark of Alzheimer's disease and is a target for molecular imaging probes to aid in diagnosis and disease monitoring. This study evaluated the feasibility of using a radiolabeled monoclonal anti-amyloid-β antibody (JRF/AβN/25) to non-invasively assess amyloid-β burden in aged transgenic mice (APPPS1-21) with μPET imaging.
Methods: We investigated the antibody JRF/AβN/25 that binds to full-length Aβ. JRF/AβN/25 was radiolabeled with a [(89)Zr]-desferal chelate and intravenously injected into 12-13 month aged APPPS1-21 mice and their wild-type (WT) controls. Mice underwent in vivo μPET imaging at 2, 4, and 7 days post injection and were sacrificed at the end of each time point to assess brain penetrance, plaque labeling, biodistribution, and tracer stability. To confirm imaging specificity we also evaluated brain uptake of a non-amyloid targeting [(89)Zr]-labeled antibody (trastuzumab) as a negative control, additionally we performed a competitive blocking study with non-radiolabeled Df-Bz-JRF/AβN/25 and finally we assessed the possible confounding effects of blood retention.
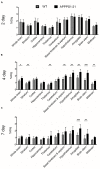
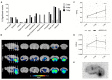
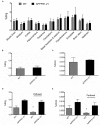
Results: Voxel-wise analysis of μPET data demonstrated significant [(89)Zr]-Df-Bz-JRF/AβN/25 retention in APPPS1-21 mice at all time points investigated. With ex vivo measures of radioactivity, significantly higher retention of [(89)Zr]-Df-Bz-JRF/AβN/25 was found at 4 and 7 days pi in APPPS1-21 mice. Despite the observed genotypic differences, comparisons with immunohistochemistry revealed that in vivo plaque labeling was low. Furthermore, pre-treatment with Df-Bz-JRF/AβN/25 only partially blocked [(89)Zr]-Df-Bz-JRF/AβN/25 uptake indicative of a high contribution of non-specific binding.
Conclusion: Amyloid plaques were detected in vivo with a radiolabeled monoclonal anti-amyloid antibody. The low brain penetrance of the antibody in addition to non-specific binding prevented an accurate estimation of plaque burden. However, it should be noted that [(89)Zr]-Df-Bz-JRF/AβN/25 nevertheless demonstrated in vivo binding and strategies to increase brain penetrance would likely achieve better results.
Keywords: 89Zirconium; Alzheimer; amyloid imaging; monoclonal antibody; small animal imaging.
Figures


References
-
- Fissers J., Waldron A.-M., De vijlder T., van Broeck B., Pemberton D. J., Mercken M., et al. (2016). Synthesis and evaluation of a Zr-89-labeled monoclonal antibody for immuno-PET imaging of amyloid-β deposition in the Brain. Mol. Imaging Biol. 10.1007/s11307-016-0935-z [Epub ahead of print]. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

