Amyloid precursor protein-mediated endocytic pathway disruption induces axonal dysfunction and neurodegeneration
- PMID: 27064279
- PMCID: PMC4855914
- DOI: 10.1172/JCI82409
Amyloid precursor protein-mediated endocytic pathway disruption induces axonal dysfunction and neurodegeneration
Abstract
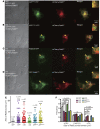
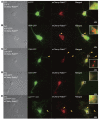
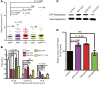
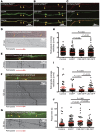
The endosome/lysosome pathway is disrupted early in the course of both Alzheimer's disease (AD) and Down syndrome (DS); however, it is not clear how dysfunction in this pathway influences the development of these diseases. Herein, we explored the cellular and molecular mechanisms by which endosomal dysfunction contributes to the pathogenesis of AD and DS. We determined that full-length amyloid precursor protein (APP) and its β-C-terminal fragment (β-CTF) act though increased activation of Rab5 to cause enlargement of early endosomes and to disrupt retrograde axonal trafficking of nerve growth factor (NGF) signals. The functional impacts of APP and its various products were investigated in PC12 cells, cultured rat basal forebrain cholinergic neurons (BFCNs), and BFCNs from a mouse model of DS. We found that the full-length wild-type APP (APPWT) and β-CTF both induced endosomal enlargement and disrupted NGF signaling and axonal trafficking. β-CTF alone induced atrophy of BFCNs that was rescued by the dominant-negative Rab5 mutant, Rab5S34N. Moreover, expression of a dominant-negative Rab5 construct markedly reduced APP-induced axonal blockage in Drosophila. Therefore, increased APP and/or β-CTF impact the endocytic pathway to disrupt NGF trafficking and signaling, resulting in trophic deficits in BFCNs. Our data strongly support the emerging concept that dysregulation of Rab5 activity contributes importantly to early pathogenesis of AD and DS.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

