A Targeted Inhibitor of the Alternative Complement Pathway Accelerates Recovery From Smoke-Induced Ocular Injury
- PMID: 27064393
- PMCID: PMC4829088
- DOI: 10.1167/iovs.15-18471
A Targeted Inhibitor of the Alternative Complement Pathway Accelerates Recovery From Smoke-Induced Ocular Injury
Abstract
Purpose: Morphologic and genetic evidence exists that an overactive complement system driven by the complement alternative pathway (AP) is involved in pathogenesis of age-related macular degeneration (AMD). Smoking is the only modifiable risk factor for AMD. As we have shown that smoke-related ocular pathology can be prevented in mice that lack an essential activator of AP, we ask here whether this pathology can be reversed by increasing inhibition in AP.
Methods: Mice were exposed to either cigarette smoke (CS) or filtered air (6 hours/day, 5 days/week, 6 months). Smoke-exposed animals were then treated with the AP inhibitor (CR2-fH) or vehicle control (PBS) for the following 3 months. Spatial frequency and contrast sensitivity were assessed by optokinetic response paradigms at 6 and 9 months; additional readouts included assessment of retinal morphology by electron microscopy (EM) and gene expression analysis by quantitative RT-PCR.
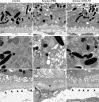
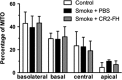
Results: The CS mice treated with CR2-fH showed significant improvement in contrast threshold compared to PBS-treated mice, whereas spatial frequency was unaffected by CS or pharmacologic intervention. Treatment with CR2-fH in CS animals reversed thinning of the retina observed in PBS-treated mice as analyzed by spectral-domain optical coherence tomography, and reversed most morphologic changes in RPE and Bruch's membrane seen in CS animals by EM.
Conclusions: Taken together, these findings suggest that AP inhibitors not only prevent, but have the potential to accelerate the clearance of complement-mediated ocular injury. Improving our understanding of the regulation of the AP is paramount to developing novel treatment approaches for AMD.
Figures



References
-
- Wong WL,, Su X,, Li X,, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014; 2: e106–e116. - PubMed
-
- Freund KB,, Zweifel SA,, Engelbert M. Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina. 2010; 30: 1333–1349. - PubMed
-
- Brown MM,, Brown GC,, Stein JD,, Roth Z,, Campanella J,, Beauchamp GR. Age-related macular degeneration: economic burden and value-based medicine analysis. Can J Ophthalmol. 2005; 40: 277–287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY014800/EY/NEI NIH HHS/United States
- R01 EY019320/EY/NEI NIH HHS/United States
- EY015128/EY/NEI NIH HHS/United States
- I01 BX003050/BX/BLRD VA/United States
- R01 EY024581/EY/NEI NIH HHS/United States
- I01 RX000444/RX/RRD VA/United States
- R01 EY015128/EY/NEI NIH HHS/United States
- C06 RR015455/RR/NCRR NIH HHS/United States
- R01EY019320/EY/NEI NIH HHS/United States
- R01 EY002576/EY/NEI NIH HHS/United States
- EY02576/EY/NEI NIH HHS/United States
- R01 NHLBI 091944/PHS HHS/United States
- P30 EY014800/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

