Expansion microscopy with conventional antibodies and fluorescent proteins
- PMID: 27064647
- PMCID: PMC4929147
- DOI: 10.1038/nmeth.3833
Expansion microscopy with conventional antibodies and fluorescent proteins
Abstract
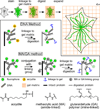
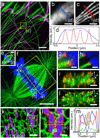
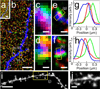
Expansion microscopy is a technique in which fluorophores on fixed specimens are linked to a swellable polymer that is physically expanded to enable super-resolution microscopy with ordinary microscopes. We have developed and characterized new methods for linking fluorophores to the polymer that now enable expansion microscopy with conventional fluorescently labeled antibodies and fluorescent proteins. Our methods simplify the procedure and expand the palette of compatible labels, allowing rapid dissemination of the technique.
Figures
Comment in
-
Expansion microscopy passes its first test.Nat Methods. 2016 May 31;13(6):481-2. doi: 10.1038/nmeth.3872. Nat Methods. 2016. PMID: 27243471 No abstract available.
-
Super-resolution microscopy writ large.Nat Biotechnol. 2016 Sep 8;34(9):928-30. doi: 10.1038/nbt.3669. Nat Biotechnol. 2016. PMID: 27606457 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

