The cAMP Pathway as Therapeutic Target in Autoimmune and Inflammatory Diseases
- PMID: 27065076
- PMCID: PMC4814577
- DOI: 10.3389/fimmu.2016.00123
The cAMP Pathway as Therapeutic Target in Autoimmune and Inflammatory Diseases
Abstract
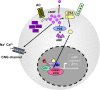
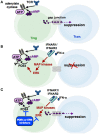
Nucleotide signaling molecules contribute to the regulation of cellular pathways. In the immune system, cyclic adenosine monophosphate (cAMP) is well established as a potent regulator of innate and adaptive immune cell functions. Therapeutic strategies to interrupt or enhance cAMP generation or effects have immunoregulatory potential in autoimmune and inflammatory disorders. Here, we provide an overview of the cyclic AMP axis and its role as a regulator of immune functions and discuss the clinical and translational relevance of interventions with these processes.
Keywords: T cells; T regulatory cells; Tregs; autoimmunity; cyclic AMP; inflammation; targeted therapies.
Figures


References
-
- Sutherland EW, Rall TW. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J Biol Chem (1958) 232:1077–91. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

