Response to Gaseous NO2 Air Pollutant of P. fluorescens Airborne Strain MFAF76a and Clinical Strain MFN1032
- PMID: 27065229
- PMCID: PMC4814523
- DOI: 10.3389/fmicb.2016.00379
Response to Gaseous NO2 Air Pollutant of P. fluorescens Airborne Strain MFAF76a and Clinical Strain MFN1032
Abstract
Human exposure to nitrogen dioxide (NO2), an air pollutant of increasing interest in biology, results in several toxic effects to human health and also to the air microbiota. The aim of this study was to investigate the bacterial response to gaseous NO2. Two Pseudomonas fluorescens strains, namely the airborne strain MFAF76a and the clinical strain MFN1032 were exposed to 0.1, 5, or 45 ppm concentrations of NO2, and their effects on bacteria were evaluated in terms of motility, biofilm formation, antibiotic resistance, as well as expression of several chosen target genes. While 0.1 and 5 ppm of NO2did not lead to any detectable modification in the studied phenotypes of the two bacteria, several alterations were observed when the bacteria were exposed to 45 ppm of gaseous NO2. We thus chose to focus on this high concentration. NO2-exposed P. fluorescens strains showed reduced swimming motility, and decreased swarming in case of the strain MFN1032. Biofilm formed by NO2-treated airborne strain MFAF76a showed increased maximum thickness compared to non-treated cells, while NO2 had no apparent effect on the clinical MFN1032 biofilm structure. It is well known that biofilm and motility are inversely regulated by intracellular c-di-GMP level. The c-di-GMP level was however not affected in response to NO2 treatment. Finally, NO2-exposed P. fluorescens strains were found to be more resistant to ciprofloxacin and chloramphenicol. Accordingly, the resistance nodulation cell division (RND) MexEF-OprN efflux pump encoding genes were highly upregulated in the two P. fluorescens strains. Noticeably, similar phenotypes had been previously observed following a NO treatment. Interestingly, an hmp-homolog gene in P. fluorescens strains MFAF76a and MFN1032 encodes a NO dioxygenase that is involved in NO detoxification into nitrites. Its expression was upregulated in response to NO2, suggesting a possible common pathway between NO and NO2 detoxification. Taken together, our study provides evidences for the bacterial response to NO2 toxicity.
Keywords: Pseudomonas fluorescens; air pollution; airborne; antibiotic sensitivity; biofilm; motility; nitrogen dioxide.
Figures
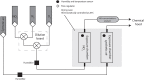

 ) and 45 ppm of NO2 treated (
) and 45 ppm of NO2 treated ( ) MFAF76a and MFN1032. Obtained results are presented as average values ± SEM. Statistical significance was calculated by the non-parametric Mann-Whitney-Test. n.s., non-significant.
) MFAF76a and MFN1032. Obtained results are presented as average values ± SEM. Statistical significance was calculated by the non-parametric Mann-Whitney-Test. n.s., non-significant.
 ). Swimming (A) and swarming (B) motilities were assayed on DMB-swim/swarm plates after 24 h incubation. The motile bacterial movement was evaluated in three independent experiments with three replicates. The data were compared with control exposed to synthetic air (
). Swimming (A) and swarming (B) motilities were assayed on DMB-swim/swarm plates after 24 h incubation. The motile bacterial movement was evaluated in three independent experiments with three replicates. The data were compared with control exposed to synthetic air ( ). Obtained results are presented as average values ± SEM. Statistical significance was calculated by the non-parametric Mann-Whitney-Test p < 0.05 (*) and < 0.001 (***).
). Obtained results are presented as average values ± SEM. Statistical significance was calculated by the non-parametric Mann-Whitney-Test p < 0.05 (*) and < 0.001 (***).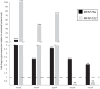
 ) and clinical MFN1032 (
) and clinical MFN1032 ( ) P. fluorescens. The GenBank accession numbers of nucleotide sequences are listed in Table S2. Quantification of mRNA level was assayed using qRT-PCR on RNAs extracted from NO2- and synthetic air- exposed P. fluorescens. The PCR reactions were performed in triplicate and the standard deviations were lower than 0.15 Ct. Statistical analysis used pairwise strain comparisons (t-test) p < 0.01 (**) and < 0.001 (***). Dotted line shows the gene expression in synthetic air- exposed control.
) P. fluorescens. The GenBank accession numbers of nucleotide sequences are listed in Table S2. Quantification of mRNA level was assayed using qRT-PCR on RNAs extracted from NO2- and synthetic air- exposed P. fluorescens. The PCR reactions were performed in triplicate and the standard deviations were lower than 0.15 Ct. Statistical analysis used pairwise strain comparisons (t-test) p < 0.01 (**) and < 0.001 (***). Dotted line shows the gene expression in synthetic air- exposed control.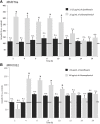
 ) and chloramphenicol (
) and chloramphenicol ( ) was assayed. Growth curves were performed with ciprofloxacin (3.125 μg/mL for MFAF76a and 1.156 μg/mL for MFN1032) and chloramphenicol (25 and 100 μg/mL respectively), and A580 was recorded at the indicated time points. The control sample was bacteria exposed to synthetic air, and grown in presence of antibiotics in indicated concentrations. The data are shown as percentages of growth relative to synthetic air-exposed control. Pooled data from three independent experiments in duplicate ± SEM are reported. Statistical significance was calculated by the non-parametric Mann-Whitney-Test p < 0.05 (*); n.s., non-significant. Dotted line shows the control (100%).
) was assayed. Growth curves were performed with ciprofloxacin (3.125 μg/mL for MFAF76a and 1.156 μg/mL for MFN1032) and chloramphenicol (25 and 100 μg/mL respectively), and A580 was recorded at the indicated time points. The control sample was bacteria exposed to synthetic air, and grown in presence of antibiotics in indicated concentrations. The data are shown as percentages of growth relative to synthetic air-exposed control. Pooled data from three independent experiments in duplicate ± SEM are reported. Statistical significance was calculated by the non-parametric Mann-Whitney-Test p < 0.05 (*); n.s., non-significant. Dotted line shows the control (100%).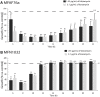
 ) and kanamycin (3.1 μg/mL;
) and kanamycin (3.1 μg/mL;  ) was tested. A580 was recorded at indicated time points. The control sample was bacteria exposed to synthetic air, and grown in presence of antibiotics in indicated concentrations. The data are presented as percentages of growth relative to air-exposed control. Pooled data from three independent experiments in duplicate ± SEM are reported. Statistical significance was calculated by the non-parametric Mann-Whitney-Test p < 0.05 (*), < 0.01 (**); n.s. non-significant. Dotted line shows the control (100%).
) was tested. A580 was recorded at indicated time points. The control sample was bacteria exposed to synthetic air, and grown in presence of antibiotics in indicated concentrations. The data are presented as percentages of growth relative to air-exposed control. Pooled data from three independent experiments in duplicate ± SEM are reported. Statistical significance was calculated by the non-parametric Mann-Whitney-Test p < 0.05 (*), < 0.01 (**); n.s. non-significant. Dotted line shows the control (100%).
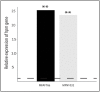
 ) and clinical MFN1032 (
) and clinical MFN1032 ( ). The GenBank accession numbers of hmp nucleotide sequences are listed in Table S2. Quantification of mRNA level was assayed using qRT-PCR on RNAs extracted from NO and synthetic air-exposed P. fluorescens. The PCR reactions were performed in triplicate and the standard deviations were lower than 0.15 Ct. Statistical analysis used pairwise strain comparisons (t-test) p < 0.01 (**). Dotted line shows the gene expression in air-exposed control.
). The GenBank accession numbers of hmp nucleotide sequences are listed in Table S2. Quantification of mRNA level was assayed using qRT-PCR on RNAs extracted from NO and synthetic air-exposed P. fluorescens. The PCR reactions were performed in triplicate and the standard deviations were lower than 0.15 Ct. Statistical analysis used pairwise strain comparisons (t-test) p < 0.01 (**). Dotted line shows the gene expression in air-exposed control.Similar articles
-
Detoxification Response of Pseudomonas fluorescens MFAF76a to Gaseous Pollutants NO2 and NO.Microorganisms. 2022 Aug 5;10(8):1576. doi: 10.3390/microorganisms10081576. Microorganisms. 2022. PMID: 36013994 Free PMC article.
-
Gaseous NO2 induces various envelope alterations in Pseudomonas fluorescens MFAF76a.Sci Rep. 2022 May 20;12(1):8528. doi: 10.1038/s41598-022-11606-w. Sci Rep. 2022. PMID: 35595726 Free PMC article.
-
Phenotypic variation in the Pseudomonas fluorescens clinical strain MFN1032.Res Microbiol. 2009 Jun;160(5):337-44. doi: 10.1016/j.resmic.2009.04.004. Epub 2009 May 4. Res Microbiol. 2009. PMID: 19409488
-
The pathogenic potential of Pseudomonas fluorescens MFN1032 on enterocytes can be modulated by serotonin, substance P and epinephrine.Arch Microbiol. 2015 Oct;197(8):983-90. doi: 10.1007/s00203-015-1135-y. Epub 2015 Jul 15. Arch Microbiol. 2015. PMID: 26175088
-
Critical review of the human data on short-term nitrogen dioxide (NO2) exposures: evidence for NO2 no-effect levels.Crit Rev Toxicol. 2009;39(9):743-81. doi: 10.3109/10408440903294945. Crit Rev Toxicol. 2009. PMID: 19852560 Review.
Cited by
-
Detoxification Response of Pseudomonas fluorescens MFAF76a to Gaseous Pollutants NO2 and NO.Microorganisms. 2022 Aug 5;10(8):1576. doi: 10.3390/microorganisms10081576. Microorganisms. 2022. PMID: 36013994 Free PMC article.
-
Gaseous NO2 induces various envelope alterations in Pseudomonas fluorescens MFAF76a.Sci Rep. 2022 May 20;12(1):8528. doi: 10.1038/s41598-022-11606-w. Sci Rep. 2022. PMID: 35595726 Free PMC article.
-
Flavohaemoglobin: the pre-eminent nitric oxide-detoxifying machine of microorganisms.F1000Res. 2020 Jan 8;9:F1000 Faculty Rev-7. doi: 10.12688/f1000research.20563.1. eCollection 2020. F1000Res. 2020. PMID: 31956400 Free PMC article. Review.
-
Deleterious Effects of an Air Pollutant (NO2) on a Selection of Commensal Skin Bacterial Strains, Potential Contributor to Dysbiosis?Front Microbiol. 2020 Dec 8;11:591839. doi: 10.3389/fmicb.2020.591839. eCollection 2020. Front Microbiol. 2020. PMID: 33363523 Free PMC article.
-
Microbial ecology of the atmosphere.FEMS Microbiol Rev. 2022 Jul 1;46(4):fuac009. doi: 10.1093/femsre/fuac009. FEMS Microbiol Rev. 2022. PMID: 35137064 Free PMC article. Review.
References
-
- Ahern H. E., Walsh K. A., Hill T. C. J., Moffett B. F. (2007). Fluorescent pseudomonads isolated from Hebridean cloud and rain water produce biosurfactants but do not cause ice nucleation. Biogeosciences 4, 115–124. 10.5194/bg-4-115-2007 - DOI
-
- Arai H., Hayashi M., Kuroi A., Ishii M., Igarashi Y. (2005). Transcriptional regulation of the flavohemoglobin gene for aerobic nitric oxide detoxification by the second nitric oxide-responsive regulator of Pseudomonas aeruginosa. J. Bacteriol. 187, 3960–3968. 10.1128/JB.187.12.3960-3968.2005 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

