Computational and Empirical Studies Predict Mycobacterium tuberculosis-Specific T Cells as a Biomarker for Infection Outcome
- PMID: 27065304
- PMCID: PMC4827839
- DOI: 10.1371/journal.pcbi.1004804
Computational and Empirical Studies Predict Mycobacterium tuberculosis-Specific T Cells as a Biomarker for Infection Outcome
Abstract
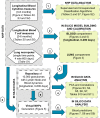
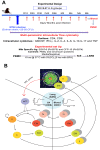
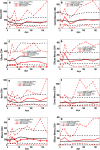
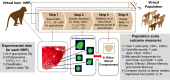
Identifying biomarkers for tuberculosis (TB) is an ongoing challenge in developing immunological correlates of infection outcome and protection. Biomarker discovery is also necessary for aiding design and testing of new treatments and vaccines. To effectively predict biomarkers for infection progression in any disease, including TB, large amounts of experimental data are required to reach statistical power and make accurate predictions. We took a two-pronged approach using both experimental and computational modeling to address this problem. We first collected 200 blood samples over a 2- year period from 28 non-human primates (NHP) infected with a low dose of Mycobacterium tuberculosis. We identified T cells and the cytokines that they were producing (single and multiple) from each sample along with monkey status and infection progression data. Machine learning techniques were used to interrogate the experimental NHP datasets without identifying any potential TB biomarker. In parallel, we used our extensive novel NHP datasets to build and calibrate a multi-organ computational model that combines what is occurring at the site of infection (e.g., lung) at a single granuloma scale with blood level readouts that can be tracked in monkeys and humans. We then generated a large in silico repository of in silico granulomas coupled to lymph node and blood dynamics and developed an in silico tool to scale granuloma level results to a full host scale to identify what best predicts Mycobacterium tuberculosis (Mtb) infection outcomes. The analysis of in silico blood measures identifies Mtb-specific frequencies of effector T cell phenotypes at various time points post infection as promising indicators of infection outcome. We emphasize that pairing wetlab and computational approaches holds great promise to accelerate TB biomarker discovery.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Low Levels of T Cell Exhaustion in Tuberculous Lung Granulomas.Infect Immun. 2018 Aug 22;86(9):e00426-18. doi: 10.1128/IAI.00426-18. Print 2018 Sep. Infect Immun. 2018. PMID: 29891540 Free PMC article.
-
A computational model tracks whole-lung Mycobacterium tuberculosis infection and predicts factors that inhibit dissemination.PLoS Comput Biol. 2020 May 20;16(5):e1007280. doi: 10.1371/journal.pcbi.1007280. eCollection 2020 May. PLoS Comput Biol. 2020. PMID: 32433646 Free PMC article.
-
Spatial Organization and Recruitment of Non-Specific T Cells May Limit T Cell-Macrophage Interactions Within Mycobacterium tuberculosis Granulomas.Front Immunol. 2021 Jan 20;11:613638. doi: 10.3389/fimmu.2020.613638. eCollection 2020. Front Immunol. 2021. PMID: 33552077 Free PMC article.
-
Genome wide approaches discover novel Mycobacterium tuberculosis antigens as correlates of infection, disease, immunity and targets for vaccination.Semin Immunol. 2018 Oct;39:88-101. doi: 10.1016/j.smim.2018.07.001. Epub 2018 Jul 7. Semin Immunol. 2018. PMID: 30327124 Review.
-
Understanding and overcoming the barriers to T cell-mediated immunity against tuberculosis.Semin Immunol. 2014 Dec;26(6):578-87. doi: 10.1016/j.smim.2014.10.003. Epub 2014 Oct 28. Semin Immunol. 2014. PMID: 25453230 Free PMC article. Review.
Cited by
-
Model-Driven Experimentation: A New Approach to Understand Mechanisms of Tertiary Lymphoid Tissue Formation, Function, and Therapeutic Resolution.Front Immunol. 2017 Apr 4;7:658. doi: 10.3389/fimmu.2016.00658. eCollection 2016. Front Immunol. 2017. PMID: 28421068 Free PMC article.
-
Heterogeneity in tuberculosis.Nat Rev Immunol. 2017 Nov;17(11):691-702. doi: 10.1038/nri.2017.69. Epub 2017 Jul 24. Nat Rev Immunol. 2017. PMID: 28736436 Free PMC article. Review.
-
Do Xpert MTB/RIF Cycle Threshold Values Provide Information about Patient Delays for Tuberculosis Diagnosis?PLoS One. 2016 Sep 9;11(9):e0162833. doi: 10.1371/journal.pone.0162833. eCollection 2016. PLoS One. 2016. PMID: 27611466 Free PMC article.
-
Multiscale Agent-Based and Hybrid Modeling of the Tumor Immune Microenvironment.Processes (Basel). 2019 Jan;7(1):37. doi: 10.3390/pr7010037. Epub 2019 Jan 13. Processes (Basel). 2019. PMID: 30701168 Free PMC article.
-
PET CT Identifies Reactivation Risk in Cynomolgus Macaques with Latent M. tuberculosis.PLoS Pathog. 2016 Jul 5;12(7):e1005739. doi: 10.1371/journal.ppat.1005739. eCollection 2016 Jul. PLoS Pathog. 2016. PMID: 27379816 Free PMC article.
References
-
- WHO (2014) Global tuberculosis report 2014. World Health Organization.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

