Loss of Keratin 17 induces tissue-specific cytokine polarization and cellular differentiation in HPV16-driven cervical tumorigenesis in vivo
- PMID: 27065324
- PMCID: PMC5333940
- DOI: 10.1038/onc.2016.102
Loss of Keratin 17 induces tissue-specific cytokine polarization and cellular differentiation in HPV16-driven cervical tumorigenesis in vivo
Abstract
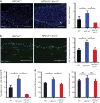
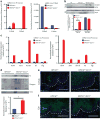
Despite preventive human papilloma virus (HPV) vaccination efforts, cervical cancer remains a leading cause of death in women worldwide. Development of therapeutic approaches for cervical cancer are hampered by a lack of mechanistic insight during tumorigenesis. The cytoskeletal protein Keratin 17 (KRT17;K17) is robustly expressed in a broad array of carcinomas, including in cervical tumors, where it has both diagnostic and prognostic value. In this study, we have established multiple functional roles for K17 in the promotion of cervical tumorigenesis in vivo using the established HPV16tg mouse model for cervical squamous cell carcinoma. In HPV16tg/+;Krt17-/-relative to HPV16tg/+ reference female mice, onset of cervical lesions is delayed and closely paralleled by marked reductions in hyperplasia, dysplasia and vascularization. In addition, loss of Krt17 is associated with a cytokine polarization and recruitment of effector immune cells to lesion-prone cervical epithelia. Further, we observed marked enhancement of terminal differentiation in HPV16tg/+;Krt17-/-cervical epithelium accompanied by a stimulation and expansion in the expression of p63, a known basal/reserve cell marker in this tissue. Altogether, the data suggest that the loss of Krt17 may foster an overall protective environment for lesion-prone cervical tissue. In addition to providing new insights into the immunomodulatory and cellular mechanisms of cervical tumorigenesis, these findings may help guide the development of future therapies including vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Zinc finger nucleases targeting the human papillomavirus E7 oncogene induce E7 disruption and a transformed phenotype in HPV16/18-positive cervical cancer cells.Clin Cancer Res. 2014 Dec 15;20(24):6495-503. doi: 10.1158/1078-0432.CCR-14-0250. Epub 2014 Oct 21. Clin Cancer Res. 2014. PMID: 25336692
-
Development and assessment of a general theory of cervical carcinogenesis utilizing a severe combined immunodeficiency murine-human xenograft model.Gynecol Oncol. 2000 Apr;77(1):137-48. doi: 10.1006/gyno.2000.5729. Gynecol Oncol. 2000. PMID: 10739703
-
Expression of the E5 Oncoprotein of HPV16 Impacts on the Molecular Profiles of EMT-Related and Differentiation Genes in Ectocervical Low-Grade Lesions.Int J Mol Sci. 2021 Jun 18;22(12):6534. doi: 10.3390/ijms22126534. Int J Mol Sci. 2021. PMID: 34207106 Free PMC article.
-
Proteomics strategies to analyze HPV-transformed cells: relevance to cervical cancer.Expert Rev Proteomics. 2013 Oct;10(5):461-72. doi: 10.1586/14789450.2013.842469. Expert Rev Proteomics. 2013. PMID: 24117203 Review.
-
Cellular and molecular alterations in human epithelial cells transformed by recombinant human papillomavirus DNA.Crit Rev Oncog. 1993;4(4):337-60. Crit Rev Oncog. 1993. PMID: 8394744 Review.
Cited by
-
Molecular iodine inhibits the expression of stemness markers on cancer stem-like cells of established cell lines derived from cervical cancer.BMC Cancer. 2018 Sep 26;18(1):928. doi: 10.1186/s12885-018-4824-5. BMC Cancer. 2018. PMID: 30257666 Free PMC article.
-
microRNA-184 Induces a Commitment Switch to Epidermal Differentiation.Stem Cell Reports. 2017 Dec 12;9(6):1991-2004. doi: 10.1016/j.stemcr.2017.10.030. Epub 2017 Nov 30. Stem Cell Reports. 2017. PMID: 29198823 Free PMC article.
-
Keratins regulate colonic epithelial cell differentiation through the Notch1 signalling pathway.Cell Death Differ. 2017 Jun;24(6):984-996. doi: 10.1038/cdd.2017.28. Epub 2017 May 5. Cell Death Differ. 2017. PMID: 28475172 Free PMC article.
-
Keratin 6a reorganization for ubiquitin-proteasomal processing is a direct antimicrobial response.J Cell Biol. 2018 Feb 5;217(2):731-744. doi: 10.1083/jcb.201704186. Epub 2017 Nov 30. J Cell Biol. 2018. PMID: 29191848 Free PMC article.
-
A role for keratin 17 during DNA damage response and tumor initiation.Proc Natl Acad Sci U S A. 2021 Mar 30;118(13):e2020150118. doi: 10.1073/pnas.2020150118. Proc Natl Acad Sci U S A. 2021. PMID: 33762306 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. - PubMed
-
- Hopman AH, Smedts F, Dignef W, Ummelen M, Sonke G, Mravunac M, et al. Transition of high-grade cervical intraepithelial neoplasia to micro-invasive carcinoma is characterized by integration of HPV 16/18 and numerical chromosome abnormalities. J Pathol. 2004;202:23–33. - PubMed
-
- Hopman AH, Theelen W, Hommelberg PP, Kamps MA, Herrington CS, Morrison LE, et al. Genomic integration of oncogenic HPV and gain of the human telomerase gene TERC at 3q26 are strongly associated events in the progression of uterine cervical dysplasia to invasive cancer. J Pathol. 2006;210:412–419. - PubMed
-
- Mitchell MF, Hittelman WN, Hong WK, Lotan R, Schottenfeld D. The natural history of cervical intraepithelial neoplasia: an argument for intermediate endpoint biomarkers. Cancer Epidemiol Biomarkers Prev. 1994;3:619–626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

