Elimination of chronic lymphocytic leukemia cells in stromal microenvironment by targeting CPT with an antiangina drug perhexiline
- PMID: 27065330
- PMCID: PMC5064824
- DOI: 10.1038/onc.2016.103
Elimination of chronic lymphocytic leukemia cells in stromal microenvironment by targeting CPT with an antiangina drug perhexiline
Abstract
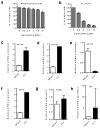
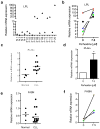
Chronic lymphocytic leukemia (CLL) is the most common adult leukemia in the western countries and is currently incurable due, in part, to difficulty in eliminating the leukemia cells protected by stromal microenvironment. Based on previous observations that CLL cells exhibit mitochondrial dysfunction and altered lipid metabolism and that carnitine palmitoyltransferases (CPT) have a major role in transporting fatty acid into mitochondria to support cancer cell metabolism, we tested several clinically relevant inhibitors of lipid metabolism for their ability to eliminate primary CLL cells. We discovered that perhexiline, an antiangina agent that inhibits CPT, was highly effective in killing CLL cells in stromal microenvironment at clinically achievable concentrations. These effective concentrations caused low toxicity to normal lymphocytes and normal stromal cells. Mechanistic study revealed that CLL cells expressed high levels of CPT1 and CPT2. Suppression of fatty acid transport into mitochondria by inhibiting CPT using perhexiline resulted in a depletion of cardiolipin, a key component of mitochondrial membranes, and compromised mitochondrial integrity, leading to rapid depolarization and massive CLL cell death. The therapeutic activity of perhexiline was further demonstrated in vivo using a CLL transgenic mouse model. Perhexiline significantly prolonged the overall animal survival by only four drug injections. Our study suggests that targeting CPT using an antiangina drug is able to effectively eliminate leukemia cells in vivo, and is a novel therapeutic strategy for potential clinical treatment of CLL.
Conflict of interest statement
The authors declare no conflict of interest
Figures




References
-
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–815. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

