Nucleos(t)ide analogues for the prevention of hepatitis B recurrence after liver transplantation do not affect serum phosphorus levels
- PMID: 27065734
- PMCID: PMC4805742
- DOI: 10.20524/aog.2016.0014
Nucleos(t)ide analogues for the prevention of hepatitis B recurrence after liver transplantation do not affect serum phosphorus levels
Abstract
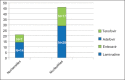
Background: Nucleos(t)ide analogues (NAs) constitute the backbone of treatment for the prevention of hepatitis B virus recurrence after liver transplantation (LT). Decline in serum phosphorus levels is a common side effect of nucleotide therapy. Our aim was to assess the impact of nucleotide treatment on the occurrence of hypophosphatemia after LT and determine possible predictors.
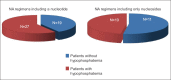
Methods: We retrospectively analyzed data from liver transplant recipients who had been transplanted for various indications. All patients were evaluated every 3 months. Each patient was considered to be having hypophosphatemia when at least one value of serum phosphorus ≤2.5 mg/dL was detected.
Results: In total, 109 patients [83 males (76%)] with a mean age of 55±10 years were included. 46/67 (67%) patients with hepatitis B received a nucleotide. The rate of hypophosphatemia (55%) was not different between patients with hepatitis B and those transplanted for other indications (62%). Patients receiving a nucleotide did not run a greater risk of hypophosphatemia than patients receiving only nucleosides (59% vs. 48%, P=0.39). Male gender and everolimus use were associated with the occurrence of hypophosphatemia in patients with hepatitis B. In multivariate analysis only gender was associated with hypophosphatemia (odds ratio 11.43, 95%CI -2.11 to -0.49; P=0.0025).
Conclusions: Hypophosphatemia occurs in more than half of liver transplant recipients regardless of the indication for LT. Male gender and everolimus use seem to predispose to hypophosphatemia, whereas the type of antiviral agent does not.
Keywords: Hepatitis B; hypophosphatemia; nucleos(t)ide; prophylaxis; transplantation.
Conflict of interest statement
Conflict of Interest: None
Figures
Similar articles
-
Nucleos(t)ide analog(s) prophylaxis after hepatitis B immunoglobulin withdrawal against hepatitis B and D recurrence after liver transplantation.Transpl Infect Dis. 2016 Oct;18(5):667-673. doi: 10.1111/tid.12575. Epub 2016 Sep 7. Transpl Infect Dis. 2016. PMID: 27421122
-
Prevention and risk factors of hepatitis B recurrence after living donor liver transplantation.J Gastroenterol Hepatol. 2014 Jan;29(1):151-6. doi: 10.1111/jgh.12403. J Gastroenterol Hepatol. 2014. PMID: 24117684
-
Treatment of chronic hepatitis B virus infection - Dutch national guidelines.Neth J Med. 2008 Jul-Aug;66(7):292-306. Neth J Med. 2008. PMID: 18663260 Review.
-
Reduction of hepatitis B surface antigen and covalently closed circular DNA by nucleos(t)ide analogues of different potency.Clin Gastroenterol Hepatol. 2013 Aug;11(8):1004-10.e1. doi: 10.1016/j.cgh.2013.01.026. Epub 2013 Feb 1. Clin Gastroenterol Hepatol. 2013. PMID: 23376799
-
The efficacy and safety of nucleos(t)ide analogues in the treatment of HBV-related acute-on-chronic liver failure: a meta-analysis.Ann Hepatol. 2013 May-Jun;12(3):364-72. Ann Hepatol. 2013. PMID: 23619252 Review.
References
-
- Cholongitas E, Papatheodoridis GV. High genetic barrier nucleos(t)ide analogue(s) for prophylaxis from hepatitis B virus recurrence after liver transplantation:a systematic review. Am J Transplant. 2013;13:353–362. - PubMed
-
- Kamar N, Huart A, Tack I, Alric L, Izopet J, Rostaing L. Renal side effects of adefovir in hepatitis B virus-(HBV) positive kidney allograft recipients. Clin Nephrol. 2009;71:36–42. - PubMed
-
- Schiff E, Lai CL, Hadziyannis S, et al. Adefovir dipivoxil for wait-listed and post-liver transplantation patients with lamivudine-resistant hepatitis B:final long-term results. Liver Transpl. 2007;13:349–360. - PubMed
-
- Heathcote EJ, Marcellin P, Buti G, et al. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology. 2011;140:132–143. - PubMed
-
- Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442–2455. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources