Electrophysiology of Hypothalamic Magnocellular Neurons In vitro: A Rhythmic Drive in Organotypic Cultures and Acute Slices
- PMID: 27065780
- PMCID: PMC4814512
- DOI: 10.3389/fnins.2016.00109
Electrophysiology of Hypothalamic Magnocellular Neurons In vitro: A Rhythmic Drive in Organotypic Cultures and Acute Slices
Abstract
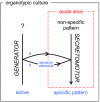
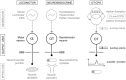
Hypothalamic neurohormones are released in a pulsatile manner. The mechanisms of this pulsatility remain poorly understood and several hypotheses are available, depending upon the neuroendocrine system considered. Among these systems, hypothalamo-neurohypophyseal magnocellular neurons have been early-considered models, as they typically display an electrical activity consisting of bursts of action potentials that is optimal for the release of boluses of the neurohormones oxytocin and vasopressin. The cellular mechanisms underlying this bursting behavior have been studied in vitro, using either acute slices of the adult hypothalamus, or organotypic cultures of neonatal hypothalamic tissue. We have recently proposed, from experiments in organotypic cultures, that specific central pattern generator networks, upstream of magnocellular neurons, determine their bursting activity. Here, we have tested whether a similar hypothesis can be derived from in vitro experiments in acute slices of the adult hypothalamus. To this aim we have screened our electrophysiological recordings of the magnocellular neurons, previously obtained from acute slices, with an analysis of autocorrelation of action potentials to detect a rhythmic drive as we recently did for organotypic cultures. This confirmed that the bursting behavior of magnocellular neurons is governed by central pattern generator networks whose rhythmic drive, and thus probably integrity, is however less satisfactorily preserved in the acute slices from adult brains.
Keywords: burst firing; oxytocin; pulse generator; supraoptic nucleus; vasopressin.
Figures





Similar articles
-
Evidence for a hypothalamic oxytocin-sensitive pattern-generating network governing oxytocin neurons in vitro.J Neurosci. 1998 Sep 1;18(17):6641-9. doi: 10.1523/JNEUROSCI.18-17-06641.1998. J Neurosci. 1998. PMID: 9712636 Free PMC article.
-
Analysis of intracellularly recorded phasic bursting by mammalian neuroendocrine cells.J Neurophysiol. 1984 Mar;51(3):552-66. doi: 10.1152/jn.1984.51.3.552. J Neurophysiol. 1984. PMID: 6321696
-
Whole-cell recordings from preoptic/hypothalamic slices reveal burst firing in gonadotropin-releasing hormone neurons identified with green fluorescent protein in transgenic mice.Endocrinology. 2000 Oct;141(10):3731-6. doi: 10.1210/endo.141.10.7690. Endocrinology. 2000. PMID: 11014229
-
Rhythmic activities of hypothalamic magnocellular neurons: autocontrol mechanisms.Biol Cell. 1997 Dec;89(9):555-60. Biol Cell. 1997. PMID: 9673007 Review.
-
Overview of cellular electrophysiological actions of vasopressin.Eur J Pharmacol. 2008 Apr 7;583(2-3):243-54. doi: 10.1016/j.ejphar.2007.11.074. Epub 2008 Jan 30. Eur J Pharmacol. 2008. PMID: 18280467 Review.
Cited by
-
The Structural and Electrophysiological Properties of Progesterone Receptor-Expressing Neurons Vary along the Anterior-Posterior Axis of the Ventromedial Hypothalamus and Undergo Local Changes across the Reproductive Cycle.eNeuro. 2021 Jun 4;8(3):ENEURO.0049-21.2021. doi: 10.1523/ENEURO.0049-21.2021. Print 2021 May-Jun. eNeuro. 2021. PMID: 33879568 Free PMC article.
-
Long-range axonal projections of transplanted mouse embryonic stem cell-derived hypothalamic neurons into adult mouse brain.PLoS One. 2022 Nov 10;17(11):e0276694. doi: 10.1371/journal.pone.0276694. eCollection 2022. PLoS One. 2022. PMID: 36356043 Free PMC article.
-
The Hypothalamic-Pituitary-Adrenal Axis: Development, Programming Actions of Hormones, and Maternal-Fetal Interactions.Front Behav Neurosci. 2021 Jan 13;14:601939. doi: 10.3389/fnbeh.2020.601939. eCollection 2020. Front Behav Neurosci. 2021. PMID: 33519393 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

