Estrogen Receptor Beta and 2-arachidonoylglycerol Mediate the Suppressive Effects of Estradiol on Frequency of Postsynaptic Currents in Gonadotropin-Releasing Hormone Neurons of Metestrous Mice: An Acute Slice Electrophysiological Study
- PMID: 27065803
- PMCID: PMC4809870
- DOI: 10.3389/fncel.2016.00077
Estrogen Receptor Beta and 2-arachidonoylglycerol Mediate the Suppressive Effects of Estradiol on Frequency of Postsynaptic Currents in Gonadotropin-Releasing Hormone Neurons of Metestrous Mice: An Acute Slice Electrophysiological Study
Abstract
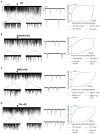
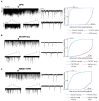
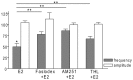
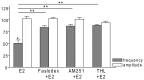
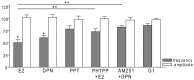
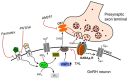
Gonadotropin-releasing hormone (GnRH) neurons are controlled by 17β-estradiol (E2) contributing to the steroid feedback regulation of the reproductive axis. In rodents, E2 exerts a negative feedback effect upon GnRH neurons throughout the estrus-diestrus phase of the ovarian cycle. The present study was undertaken to reveal the role of estrogen receptor subtypes in the mediation of the E2 signal and elucidate the downstream molecular machinery of suppression. The effect of E2 administration at low physiological concentration (10 pM) on GnRH neurons in acute brain slices obtained from metestrous GnRH-green fluorescent protein (GFP) mice was studied under paradigms of blocking or activating estrogen receptor subtypes and interfering with retrograde 2-arachidonoylglycerol (2-AG) signaling. Whole-cell patch clamp recordings revealed that E2 significantly diminished the frequency of spontaneous postsynaptic currents (sPSCs) in GnRH neurons (49.62 ± 7.6%) which effect was abolished by application of the estrogen receptor (ER) α/β blocker Faslodex (1 μM). Pretreatment of the brain slices with cannabinoid receptor type 1 (CB1) inverse agonist AM251 (1 μM) and intracellularly applied endocannabinoid synthesis blocker THL (10 μM) significantly attenuated the effect of E2 on the sPSCs. E2 remained effective in the presence of tetrodotoxin (TTX) indicating a direct action of E2 on GnRH cells. The ERβ specific agonist DPN (10 pM) also significantly decreased the frequency of miniature postsynaptic currents (mPSCs) in GnRH neurons. In addition, the suppressive effect of E2 was completely blocked by the selective ERβ antagonist PHTPP (1 μM) indicating that ERβ is required for the observed rapid effect of the E2. In contrast, the ERα agonist PPT (10 pM) or the membrane-associated G protein-coupled estrogen receptor (GPR30) agonist G1 (10 pM) had no significant effect on the frequency of mPSCs in these neurons. AM251 and tetrahydrolipstatin (THL) significantly abolished the effect of E2 whereas AM251 eliminated the action of DPN on the mPSCs. These data suggest the involvement of the retrograde endocannabinoid mechanism in the rapid direct effect of E2. These results collectively indicate that estrogen receptor beta and 2-AG/CB1 signaling mechanisms are coupled and play an important role in the mediation of the negative estradiol feedback on GnRH neurons in acute slice preparation obtained from intact, metestrous mice.
Keywords: 2-AG; CB1; GABA; GnRH neuron; estrogen receptor beta; negative estrogen feedback; retrograde signaling.
Figures
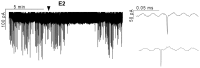


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

