Noise-Induced "Toughening" Effect in Wistar Rats: Enhanced Auditory Brainstem Responses Are Related to Calretinin and Nitric Oxide Synthase Upregulation
- PMID: 27065815
- PMCID: PMC4815363
- DOI: 10.3389/fnana.2016.00019
Noise-Induced "Toughening" Effect in Wistar Rats: Enhanced Auditory Brainstem Responses Are Related to Calretinin and Nitric Oxide Synthase Upregulation
Abstract
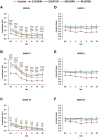
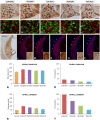
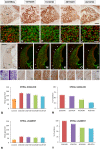
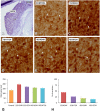
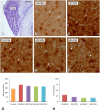
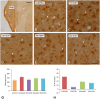
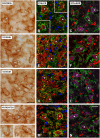
An appropriate conditioning noise exposure may reduce a subsequent noise-induced threshold shift. Although this "toughening" effect helps to protect the auditory system from a subsequent traumatic noise exposure, the mechanisms that regulate this protective process are not fully understood yet. Accordingly, the goal of the present study was to characterize physiological processes associated with "toughening" and to determine their relationship to metabolic changes in the cochlea and cochlear nucleus (CN). Auditory brainstem responses (ABR) were evaluated in Wistar rats before and after exposures to a sound conditioning protocol consisting of a broad-band white noise of 118 dB SPL for 1 h every 72 h, four times. After the last ABR evaluation, animals were perfused and their cochleae and brains removed and processed for the activity markers calretinin (CR) and neuronal nitric oxide synthase (nNOS). Toughening was demonstrated by a progressively faster recovery of the threshold shift, as well as wave amplitudes and latencies over time. Immunostaining revealed an increase in CR and nNOS levels in the spiral ganglion, spiral ligament, and CN in noise-conditioned rats. Overall, these results suggest that the protective mechanisms of the auditory toughening effect initiate in the cochlea and extend to the central auditory system. Such phenomenon might be in part related to an interplay between CR and nitric oxide signaling pathways, and involve an increased cytosolic calcium buffering capacity induced by the noise conditioning protocol.
Keywords: Wistar rats; calcium; cochlear nucleus; conditioning; priming; toughening.
Figures






References
-
- Alvarado J. C., Fuentes-Santamaría V., Franklin S. R., Brunso-Bechtold J. K., Henkel C. K. (2005). Unilateral cochlear ablation in adult ferrets results in upregulation in calretinin immunostaining in the central nucleus of the inferior colliculus. Neuroscience 136, 957–969. 10.1016/j.neuroscience.2005.04.022 - DOI - PubMed
-
- Alvarado J. C., Fuentes-Santamaría V., Franklin S. R., Brunso-Bechtold J. K., Henkel C. K. (2007a). Synaptophysin and insulin-like growth factor-1 immunostaining in the central nucleus of the inferior colliculus in adult ferrets following unilateral cochlear removal: a densitometric analysis. Synapse 61, 288–302. 10.1002/syn.20373 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

