Basic Pharmacological and Structural Evidence for Class A G-Protein-Coupled Receptor Heteromerization
- PMID: 27065866
- PMCID: PMC4815248
- DOI: 10.3389/fphar.2016.00076
Basic Pharmacological and Structural Evidence for Class A G-Protein-Coupled Receptor Heteromerization
Abstract
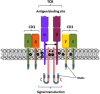
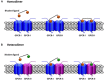
Cell membrane receptors rarely work on isolation, often they form oligomeric complexes with other receptor molecules and they may directly interact with different proteins of the signal transduction machinery. For a variety of reasons, rhodopsin-like class A G-protein-coupled receptors (GPCRs) seem an exception to the general rule of receptor-receptor direct interaction. In fact, controversy surrounds their potential to form homo- hetero-dimers/oligomers with other class A GPCRs; in a sense, the field is going backward instead of forward. This review focuses on the convergent, complementary and telling evidence showing that homo- and heteromers of class A GPCRs exist in transfected cells and, more importantly, in natural sources. It is time to decide between questioning the occurrence of heteromers or, alternatively, facing the vast scientific and technical challenges that class A receptor-dimer/oligomer existence pose to Pharmacology and to Drug Discovery.
Keywords: GPCR; dimerization; dopamine receptor; heteromer; homodimer; ligands; mammalian receptor; signal transduction taste receptor.
Figures
References
-
- Akgün E., Javed M. I., Lunzer M. M., Smeester B. A., Beitz A. J., Portoghese P. S. (2013). Ligands that interact with putative MOR-mGluR5 heteromer in mice with inflammatory pain produce potent antinociception. Proc. Natl. Acad. Sci. U.S.A. 110 11595–11599. 10.1073/pnas.1305461110 - DOI - PMC - PubMed
-
- Ballesteros J. A., Weinstein H. (1995). Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 25 366–428. 10.1016/S1043-9471(05)80049-7 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

