Dual Regulation of Cell Death and Cell Survival upon Induction of Cellular Stress by Isopimara-7,15-Dien-19-Oic Acid in Cervical Cancer, HeLa Cells In vitro
- PMID: 27065873
- PMCID: PMC4814465
- DOI: 10.3389/fphar.2016.00089
Dual Regulation of Cell Death and Cell Survival upon Induction of Cellular Stress by Isopimara-7,15-Dien-19-Oic Acid in Cervical Cancer, HeLa Cells In vitro
Abstract
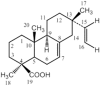
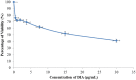
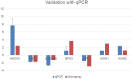
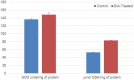
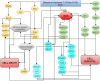
The Fritillaria imperialis is an ornamental flower that can be found in various parts of the world including Iraq, Afghanistan, Pakistan, and the Himalayas. The use of this plant as traditional remedy is widely known. This study aims to unveil the anti-cancer potentials of Isopimara-7,15-Dien-19-Oic Acid, extracted from the bulbs of F. imperialis in cervical cancer cell line, HeLa cells. Flow cytometry analysis of cell death, gene expression analysis via cDNA microarray and protein array were performed. Based on the results, Isopimara-7,15-Dien-19-Oic acid simultaneously induced cell death and promoted cell survival. The execution of apoptosis was apparent based on the flow cytometry results and regulation of both pro and anti-apoptotic genes. Additionally, the regulation of anti-oxidant genes were up-regulated especially thioredoxin, glutathione and superoxide dismutase- related genes. Moreover, the treatment also induced the activation of pro-survival heat shock proteins. Collectively, Isopimara-7,15-Dien-19-Oic Acid managed to induce cellular stress in HeLa cells and activate several anti- and pro survival pathways.
Keywords: 15-dien-19-oic acid; Fritillaria imperialis; HeLa; antioxidant; isopimara-7.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

